As the COVID-19 pandemic continues to cause economic and social disruption in addition to infections and deaths, it is slowly becoming clear that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is mutating / changing as it spreads to different regions.
A mass vaccination programme against Covid-19 is set to roll all over the world.
- While the government is working to ease the fears of those who are worried about safety, some people have a more primal fear — genetic engineered.
- The vaccines will not genetically engineer you.
- The mRNA vaccine is more like a snapchat message which once it passes its instructions to the cell, is scrapped.
- People are also fearing that the virus is mutating. Well there are already thousands of mutations which have already happened and most of them are weakening the virus as is seen in this study on various states in India.
- To calm all the above worries we have compiled here a ready guide to quell all worries about vaccines, mutations, side effects and immune memory.
- Last thing… You must celebrate the VACCINES, the greatest progress that we humans have made.
- Countries across the globe are shutting their borders to Britain due to fears about a highly infectious new coronavirus strain, causing travel chaos and raising the prospect of food shortages in the United Kingdom.
- But there is a note of calm from vaccine developers about the UK mutation echoed the World Health Organization, which cautioned against major alarm, saying this was a normal part of a pandemic’s evolution.
This trend is driven by spontaneous adaptive mutations, shaped by the different natural selection pressures ( remember Darwin ) exerted by varying environmental conditions and hosts. This has been enhanced by the adoption of lockdowns and other social distancing measures. Hence it is important to uncover the dynamics of the virus with particular attention to geographic variation.
Explained: Mutated coronavirus vs tests and vaccines
- In emerging information about the new variant of the coronavirus SARS-CoV-2 circulating in the UK, one mutation has been of particular concern. The variant, called VUI 202012/01 and reported as being capable of transmitting faster among people, is defined by as many as 14 mutations and three deletions in its genetic material. Of particular concern is one mutation, N501Y. While the variant’s potential to impact testing and vaccination results are still being studied, health authorities are largely optimistic that most tests and vaccines will still work.
What is a mutation?
- A mutation means an alteration in genetic material. In an RNA virus such as SARS-CoV-2, proteins are made of a sequence of amino acids. Such a virus contains some 30,000 ‘base pairs’, which are like bricks placed next to each other to form a structure. An alteration in this base can be a mutation, effectively changing the shape and behaviour of the virus.
- In the UK variant, one mutation has made the virus more likely to bind with human proteins called receptors. This is called N501Y.
What is N501Y?
- In simple words, the amino acid represented by the letter N, and present at position 501 in the coronavirus genetic structure, has been replaced in that position with another amino acid, represented by Y. The position where this alteration has taken place is in the spike protein’s receptor-binding domain. (It is the spike protein of the virus that binds with the human receptor.)
- Therefore, the mutation has increased the binding affinity of the coronavirus. The mutated virus reportedly accounts for 60% of recent infections in London.
- According to the Global Initiative on Sharing Avian Influenza Data (GISAID) database, the same mutation in the receptor binding domain has been independently reported in several countries including South Africa and Australia. Sequence analysis has shown that this mutation originated separately in the UK and South Africa.
What about the other coronavirus mutations?
- Mutations are common, but the majority of them cause no alteration in the structure of the proteins they encode — these are called ‘synonymous’ mutations, as they eventually translate to the same amino acids. Another type is ‘non-synonymous’ mutation, which could result in an amino acid change.
- In the variant circulating in the UK, there are six synonymous alterations and fourteen non-synonymous mutations. In addition, there are three ‘deletions’ — amino acids removed from the sequence.
- According the World Health Organization (WHO), other than N501Y, mutations that “may influence the transmissibility of the virus in humans” are P681H and HV 69/70.
And what are P681H and HV 69/70?
- P681H: This mutation has occurred in the amino acid present at 681 — another position in the receptor-binding domain. Here the amino acid P has been replaced with H. The US Centers for Disease Control (CDC) has said this is a site “with high variability in coronaviruses”, and this specific mutation has also emerged spontaneously multiple times. The WHO has said this mutation is of “biological significance”.
- Researchers have earlier shown that this mutation can promote entry into respiratory epithelial cells and transmission in animal models.
- Recent samples sequenced at the African Centre of Excellence for Genomics of Infectious Diseases, Redeemer’s University, Nigeria has shown the P681H sequence there. However, researchers say that at present, they “do not have evidence to indicate that the P681H variant is contributing to increased transmission of the virus in Nigeria”.
- HV 69/70: This mutation is the result of a deletion of amino acids at positions 69 and 70. These positions are again in the spike protein of the virus. This deletion has been observed in France and South Africa as well. The CDC has said : “This double deletion has occurred spontaneously many times, and likely leads to a change in the shape of (i.e., a conformational change in) the spike protein”.
- Researchers on behalf of COVID-19 Genomics Consortium UK (CoG-UK), which red-flagged the new variant in the UK, have said in their preliminary report that this deletion was also seen in a mink-associated outbreak in Denmark. In humans, this deletion has been associated with another mutation, N439K, which again occurred the receptor-binding domain.
- The WHO has highlighted that this deletion can affect the performance of some RT-PCR tests that detect the novel coronavirus.
How can it affect RT-PCR tests?
- The WHO has said that the deletion at positions 69/70 has been found to affect the performance of some diagnostic PCR assays that use an ‘S gene target’ (in the ‘S’ or spike protein). However, the WHO has also said, “most PCR assays worldwide use multiple targets and therefore the impact of the variant on diagnostics is not anticipated to be significant”.
- The CDC, too, has said that most commercial PCR tests have multiple targets where they detect the virus, so that even if a mutation impacts one of the targets, the other PCR targets will still work.
- In fact, infection triggered by the new strain in the UK, too, was detected by the conventional RT-PCR test.
- “Laboratories using in-house PCR assays that target the S gene of the virus should also be aware of this potential issue. In order to limit the impact on the detection capacities in the countries, an approach using different assays in parallel or multiplex assays targeting different viral genes is also recommended to allow the detection of potential arising variants,” the WHO had recommended.
Will it impact vaccine development?
- The CDC has said vaccines approved by the US Food and Drug Administration (FDA) are “polyclonal”, producing antibodies that target several parts of the spike protein.
- “The virus would likely need to accumulate multiple mutations in the spike protein to evade immunity induced by vaccines or by natural infection,” it has said.
- And the WHO has said: “Laboratory studies are ongoing to determine whether these variant viruses have different biological properties or alter vaccine efficacy. There is not enough information at present to determine if this variant is associated with any change in severity of clinical disease, antibody response or vaccine efficacy.”
Cross Species
- One development scientists are closely watching is the emergence of new strains found on mink farms in Denmark and the Netherlands that have been shown to infect humans.
- There’s work being done to confirm that at least one of those strains — the so-called cluster 5 virus — may have evolved enough changes to its spike proteins that help it escape the vaccine.
- The research needs to be verified, but early work suggests that the virus appears to have changed to help it infect minks more efficiently, while also keeping its ability to infect humans.
- When a virus evolves in a way that allows it to circulate in an animal species, “it becomes more difficult to eradicate that virus,” .
- If the virus continues to be passed in minks, if we vaccinated everyone in Denmark, but left the minks, the virus would hang out until there were enough new, susceptible hosts — typically young children — and then jump back into humans.
- For that reason, mink farms may need to take further steps, like vaccinating their animals, or, in the worst case, killing their minks, to control the spread.
The India Study
- The current study was focused on finding and interpreting the association between the evolution of the SARS-CoV-2 and the conditions in the Indian environment and hosts.
Increasing Spike Mutations, Lower Viral Fitness
- The researchers looked at how the viral spike glycoprotein showed mutation-derived differences since this is the key to viral binding, fusion, and cell entry leading to host cell infection. This is the key pathogenetic feature that differentiates it from earlier pathogenic coronaviruses SARS-CoV and MERS-CoV. It also carries the two most important genetic features of the virus.
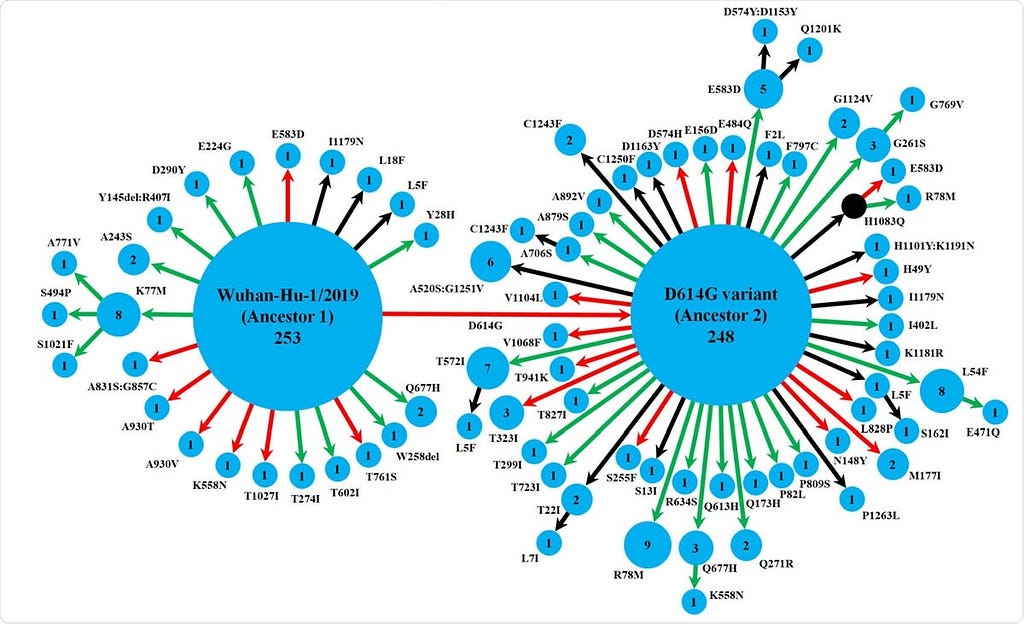
- They used the genomic sequences of 630 Indian isolates retrieved from the GISAID database. Tracing these mutations showed that the spike protein variants followed two pathways starting from two ancestral strains, namely, Wuhan-Hu-1/2019 and its D614G variant.
- While the Wuhan-Hu-1/2019 strain gave rise to the D614G variant, it also evolved to 20 other variants, of which one further led to three others. The other ancestral strain D614G gave rise to 47 variants, of which 9 led to further variants. The two ancestral strains are predominant, but there were 16 detectable spike protein variants found in multiple isolates, with different levels of spike-receptor stability. Strikingly, several mutations occurred independently at the same position across eight variants, which were not linked phylogenetically.
- Over half of the variants were found only in India, but over two-thirds had lower spike-receptor stability than the ancestral strains. This should be expected in a region where a new virus that is rapidly mutating tries to adapt to a host while doing so. Both positive and detrimental mutations may occur without reversion in the absence of any genetic recombination mechanism.
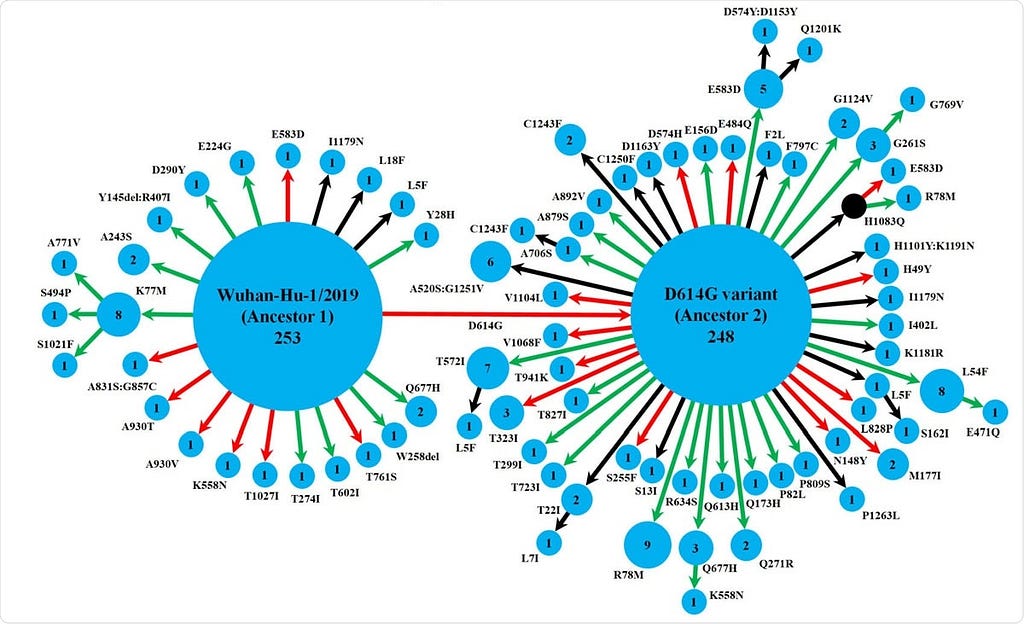
Schematic representation of the diversity of spike protein variants circulating in India, using maximum likelihood-based phylogeny reconstruction. Each node represents a specific spike protein variant, while the node-size and the number inside depict the frequency of that variant. The red or green color of each arrow indicates the higher or lower stability index respectively of the S-R complex for each variant than the major ancestral variant it emerged from (either Ancestor 1 or Ancestor 2). The black arrows lead to the variants for which the docking scores could not be determined either because of the presence of at least one variation outside the available template region for docking(18, 42), or due to non-existing isolate in the lone hypothetical node with H1083Q mutation denoted by black color. This black node signifies a variant with no available isolate in our dataset, while it gives rise to two derived variants, H1083Q:R78M and H1083Q:E583D, for which representative isolates were available.
- The implication is that this rapid buildup of mutations in the more recent strains leads to a loss of fitness compared to their ancestors, despite the constant increase in the rate of mutations. The researchers invoked Muller’s ratchet, the hypothesis that in asexual reproduction, the irreversible accumulation of harmful mutations causes a species to die out gradually.
- The interesting thing is that scientists perceive the ability of SARS-CoV-2 to jump the species barrier and infect humans to be due to specific mutations. This is an RNA virus which lends it an inherently high mutation rate. Understanding how this is occurring at the molecular level could help understand how it causes disease and how it can be curbed.
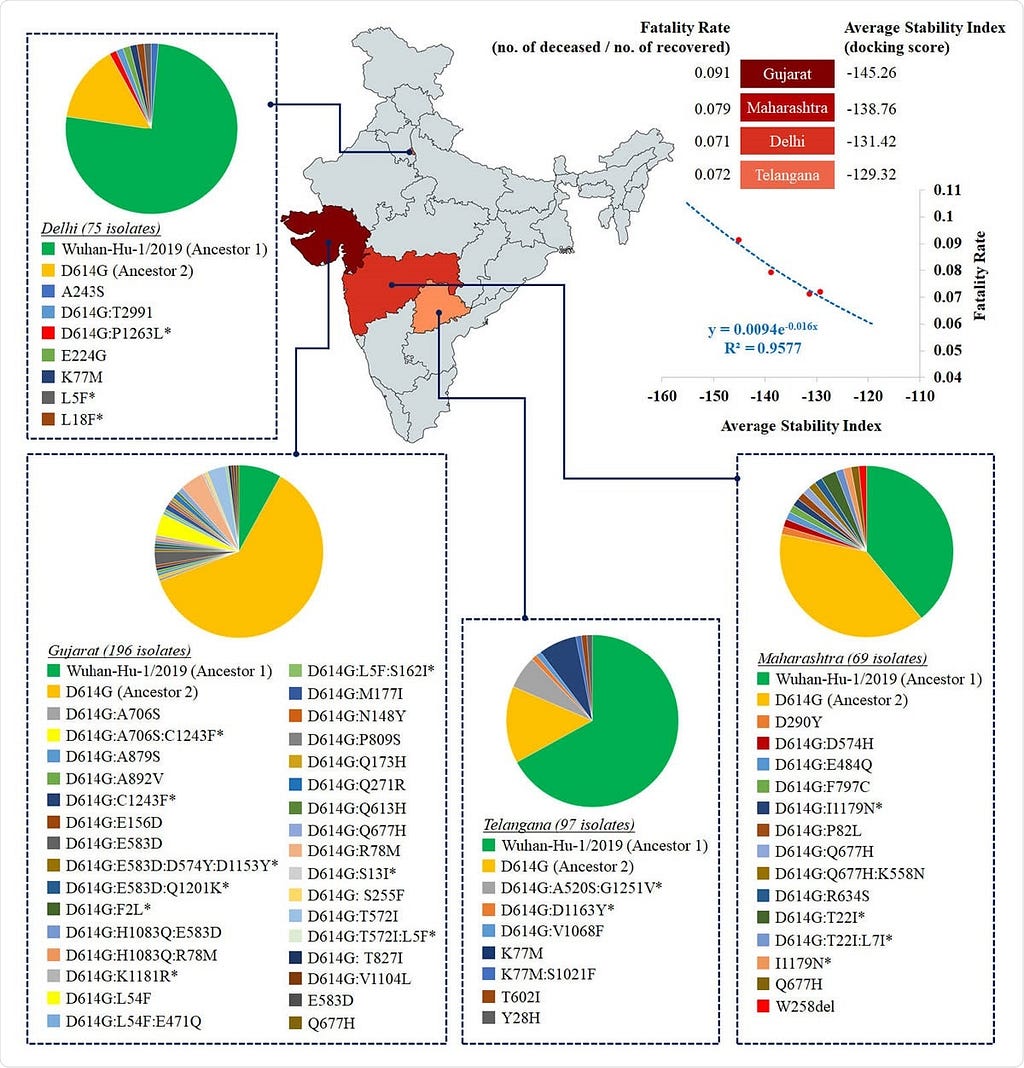
Heat map distribution across four Indian states with >50 sequenced isolates based on average stability index. The average stability index for a particular state denotes the averaged value of docking scores / HADDOCK scores of S-R complexes for all circulating variants. The values of average stability index and fatality rate in Indian states are plotted to fit an exponential function (R2=0.96).
Lower Fatality Rate with Decreasing Stability
- The most significant spike protein diversity was in Maharashtra, Odisha, West Bengal, and Gujarat, but this is limited by the fact that many other states had very few isolates. This diversity could be due to positive selection pressures enabling the protein, which is a prime immune target, to evade host immunity and allow entry into the cell. This could affect spike-receptor binding. They found 41 and 22 mutations in the S1 and S2 subunits of the spike, respectively.
- The study of mean spike-receptor complex stability in each state, based on the individual stability indices of the variants in circulation in that state, was then performed using the docking score of the S1 subunit with the receptor. Limiting it to only those with 50 or more sequenced isolates, they looked at this index in Maharashtra, Delhi, Gujarat, and Telangana, which account for 70% of the total isolates, and cover a wide range of diversity.
- The mean stability of the spike-receptor complex across these variants was strongly and exponentially correlated with fatality rates (deceased: recovered cases) across the Indian subcontinent. The fatality rate dropped by seven times between April 11 and June 28, 2020. Telangana and Delhi had similar average stabilities at a 7% fatality rate, while Maharashtra and Gujarat showed exponentially lower stabilities, with 8% and 9% fatality rates, respectively.
- This suggests, “the S-R complex stability [is] a potential marker to assess the severity of the disease.” It suggest that further study is necessary at the population level to validate this experimentally, especially since the actual parameter (the docking score) used has not been directly correlated with the fatality rate so far.
Implications
- The researchers put forth the theory that this loss of stability may be responsible for the lower national fatality rate. In India, after the initial phase in which the fatality steadily went up for four weeks, to peak at 38% on April 11, 2020, it declined sharply until it reached 5% on June 28, 2020, the date at which the researchers last obtained their data. However, the mutation rate was increasing during this period from March to May 2020.
- “Could Muller’s ratchet be a player in shaping SARS-CoV-2 evolutionary dynamics in India?” In other words, the virus could be gradually extinguishing itself under the accumulating weight of deleterious mutations, without the washout process of natural selection being able to compensate because of the rapid rate of mutations. This causes harmful mutations to be fixed in the viral population, and this buildup could lead to a “mutational meltdown.”
- The short period of the study precludes a definitive answer, but the trend may be seen, according to the researchers. This could also help evolve a better therapeutic approach, facilitating the meltdown process by inducing more harmful mutations. This will require large-scale genomic studies to answer the question, and thereby helping to improve public health surveillance and prevention programs. Some of the results of these genomic studies are compiled beneath.
The Immune Memory
Q- Do the Covid-19 vaccines deliver protective immunity
- A - Evidence supports both T and B cell responses to the three leading vaccines
- Early in the covid-19 pandemic it was unclear whether and how individuals and populations would develop protective and enduring immunity against SARS-CoV-2, either after infection or vaccination. Initial focus was on defining virus neutralising antibodies from B cells after infection. Early reports indicated that such antibodies decline substantially over less than six months, raising questions about how long protective immunity might last following infection. T cells are also known to be important in protecting against many viral infections through processes known as cellular immunity. Defining the roles of T cells in covid-19 became a central focus for investigation.
- Both memory T cell and B cell responses specific to SARS-CoV have now been found up to six months after infection. Similar T and B cell responses might be expected following vaccination, and may account for the good efficacy suggested by interim results from the three most advanced vaccine candidates. All three vaccines — two mRNA vaccines (Pfizer-BioNTech and Moderna) and a DNA vaccine (Oxford-AstraZeneca) — encode genetic information, enabling the body to produce a viral antigen (the spike protein) that stimulates an immune response. In phase I and II trials, all three vaccines induced neutralising antibodies to the spike protein and also cellular immune responses.Interim data from phase III trials suggest all three vaccines protect against symptomatic infection with SARS-CoV-2.
- Trials of the two RNA vaccines report efficacies above 90%. The viral vector DNA vaccine trial (Oxford-AstraZeneca) reported an average of 70% efficacy, ranging from 62–90% in subgroups receiving different vaccination dosages. Oxford-AstraZeneca group, Moderna group and Pfizer-BioNTech group have published their phase III trials in peer reviewed journals (Lancet and New England Journal of Medicine respectively), confirming efficacy claims made in earlier press releases. Medicines regulators in the UK and US have now approved these vaccine for emergency use after reviewing all the short term safety, efficacy, and quality data submitted by the manufacturer.
Cellular immunity
Q - What role might immune cellular responses play in the development of immunity to SARS-CoV-2 and what are the implications for vaccines?
- A - As T cells recognise and respond to viral antigens they produce many protective reactions and effector molecules. One such molecule is the cytokine interferon γ, secreted by CD4+ and CD8+ T cells and their memory cells. This can be measured in laboratory tests such as the ELISpot assay as a means of documenting specific T cell responses to viral antigens.
- Individuals with high antibody levels after infection have been shown to have a high number of SARS-CoV-2 specific T cells secreting interferon γ. T cells producing interferon γ have also been detected a median of 75 days after PCR confirmed covid-19 in people with undetectable SARS-CoV-2 antibodies, suggesting immunity is partly mediated and maintained by memory T cells. Finally, a preprint of a recent study of 100 people with a history of asymptomatic or mild covid-19 reports T cell mediated immune responses lasting for at least six months in all participants.
- These studies and others offer strong evidence that T cell immune responses are sustained, even in the face of declining or undetectable antibodies, implying that some immunity persists. It is possible, but as yet unconfirmed, that immunity might last even longer, as T cell responses to SARS-CoV-1 and MERS-CoV have been found several years after infection. The evidence from new studies, interim results from phase III vaccine trials, and previous data from phase I and phase II trials support the notion that memory T cell responses to the vaccines, along with B cell antibody responses, should provide good and possibly enduring immunity to SARS-Cov-2. This, together with continuing public health measures, should help lay a pathway out of the pandemic.
- High vaccine uptake will be critical to achieving individual and population immunity. Equitable global access to effective vaccines is also essential. Open debate and public education campaigns will be required to build trust and counter vaccine hesitancy and effective pharmacovigilance will be needed to monitor long term safety. Continued research and development will be needed to stay ahead of potentially consequential viral mutations, which could have negative consequences for covid-19 vaccines. The scientific achievements since SARS-CoV-2 was first identified less than year ago give grounds for optimism that within a reasonable timeframe we should, globally, be able to successfully manage this pandemic. We should also have learnt enough to prepare for — if not to avert — future epidemics and pandemics.
Q - Questions about immunity and vaccines include the basic question, how safe are mRNA vaccines? Do people have immune memory to this virus or not? And what does that memory look like?
A - Lets understand how our immune system reacts to this coronavirus and its implications for vaccines. But before we get to those questions, lets understand essentially the immune memory. An immune memory really is a lot like brain memory; it’s you’ve seen something before and your immune system has figured out how to recognize it and remember it. It’s really one of three major parts that you’ve got:
- Antibodies.
- Helper T cells
- Killer T cells.
And the the simple way to think about those is antibodies are really good at stopping a virus outside of cells, but once a virus has infected the cells, then you really need T cells. T cells are specialized for dealing with infected cells and antibodies get made by B cells, and so in terms of memory, you’ve really got memory B cells that can make the antibodies. You’ve got the antibodies that are actually circulating in your blood and then you’ve got these two kinds of T cells that can either kill cells or have other jobs, and so what we did was to analyzes in people who had had COVID-19, whether they have these four kinds of memory and some sub flavors of those? How much of that, and how long did it last? And the quick answer was essentially like 95 percent of people at six to eight months post-infection really had a robust amount of immune memory based on these measurements we did, and this is the largest study of immune memory ever in people to actually measure all of these different parts of immune memory. So it was a lot of work, but the results were pretty interesting that people’s immune system do tend to be remembering this virus pretty well. So that was our recent study.
Understanding SARS-CoV-2
Q - Is SARS-COV-2 made up of, 25 or 28 major proteins? The scientists at Pfizer and BioNTech and Moderna have isolated the messenger RNA for just the spike protein ? Why did both companies choose to use spike protein for their target for this vaccine?
- Spike protein is just one protein but it is a trimer, so it ends up being three copies of the same protein, so it’s all encoded by one RNA. It’s the same sequence just three folded together three times. There are about 29 licensed human vaccines, depending on how you count, and almost all of them work on the basis of protective antibody responses, and so when you’re trying to move fast with vaccine development, the most obvious target is to try and make antibodies against the protein that’s on the surface of the virus, because antibodies work by binding to the surface of a virus and essentially covering the virus and keeping the virus from doing anything. That’s really the simple way to think about antibodies working, and so for previous coronaviruses, it was known that there are a couple of different proteins on the surface of the virus, but it’s really the spike protein that’s the major one and probably the most important antibody target, and sure enough, in the months subsequent to those decisions, lots of data have accumulated that have said, essentially, all of the neutralizing antibodies, the important antibodies against SARS-CoV-2, are against the spike protein. So the spike protein is the best target to focus on for antibodies, and when we talk about three parts of the immune system, one of the concerns has been that sometimes antibodies aren’t that great at stopping all viruses, and then you really need the T cells to kick in, and the T cells don’t necessarily recognize spike. They might recognize some of those other 25 proteins, and so that was actually our first major scientific study on COVID-19 was to analyze in infected people: do people make T cells that recognize spike also or only other proteins? And what we found was that it infected people, actually people make a lot of T cell responses to spike also, and so that was really a good sign supporting the vaccine development at all that. Some viruses you have to choose more than one protein. For this virus, it looks like, “yeah just choosing one protein is a reasonable way to try and get antibody responses and T cell responses.
Q - Along those lines, if this virus mutated, well we know it’s mutating all the time, but if there was a mutation, is it possible that there’s a mutation where this virus could infect and can cause harm without the spike protein?
- A - No, not without the spike protein. The question’s really about whether you know if this is the spike protein. Can it mutate the spike protein so it looks a little bit different and now antibodies are recognizing the three-dimensional structure they’re physically binding? It’s sort of like if, it’s like anything. It’s like ,you know, it’s like my mouth, right? And it’s like, well, maybe the antibodies are really recognizing this little knobby wheel, and if they’re just recognizing that and the virus mutates that, well now you’re in trouble, because you’re not seeing the other parts of it, and so that’s something people have been spending a lot of attention to. Of where exactly are those virus mutations? And then, where are the antibody responses people are making? And viruses behave different ways, so flu is a really big problem in that way, where flu is clearly able to mutate, but lots of other viruses aren’t. So like measles, there’s been a measles vaccine for, what, 70 years now? And the virus has never managed to mutate away from that. And same thing with polio and hepatitis B, and so far it looks like 95 percent of people still had antibodies that neutralize that virus very well, and that’s probably because every single person is making multiple different antibodies, so even if the virus has one mutation, that doesn’t escape, because it’s only escaping one little part of the immune response.
Q - Scientists have been able to isolate this one strand of mRNA that just codes for the spike protein, and then they’ve packaged it into what are called lipid nanoparticles. Basically just little fat droplets. Very small microscopic fat droplets. Super tiny butter droplets. So, why do they package it that way and also how do they package that way? They have this mRNA, how do they get it into the lipid nanoparticle?
- A — For that we also need to deal with just what is RNA, and why is an RNA vaccine a reasonable approach? So RNA is a really common molecule in your body; essentially all living things use RNA as messages, and those messages encode within a cell. At any one of your cells, at any given time, you’ve got like 5,000 different RNAs and those RNAs are each encoding different messages that tell the cells to do different things, make different proteins, and RNAs are made to be transient, so they’re really a lot like, it’s like 5,000 Post-It notes, and they’ll be around for minutes or hours, and then they get shredded up, and they’re gone, they’re temporary, and so an RNA vaccine is same thing, it’s a temporary message, but it has to get into the cell, and so if it’s in the cell, the cell will now read that message and do what the message says, which helps then instruct the immune system. And then the message goes away okay. So RNA are these temporary messages, or like snapchat messages is the other analogy. There’s a message and then it it expires. Technologically one of the big challenges there is that RNA is temporary, it gets shredded up really easily — again like just shredding up a Post-It note — and so you got to get it into the cells without it all getting shredded up. So if you just inject RNA from a syringe into somebody’s skin, it doesn’t get into the cells. So the trick that people figured out over the past 10 years was, “you can put it in these little butter droplets, and those little droplets will basically fuse with the cells and release the RNA into the cell.” So now you’ve got, the message has now made it into the cell where it needs to be read, and then it can be shredded up afterwards. So it’s just, it’s a delivery system to get them all get the RNA into the cells.
Q - The lipid nanoparticle that’s taken this mRNA vaccine, what cells in our body does it actually go into? Is it just muscle cells in our arm where we get the injection?
- A - So it definitely goes into muscle cells and scientists are still learning which cells are the important cells, basically. Most of the cells that are getting the RNA are the muscle cells, and it’s possible that specialized cells of the immune system that aren’t very common, but they may get the RNA, and those may be the more important for starting the immune response, but yeah most of the RNA is going into the muscle cells and I’m sure the protein expression there matters. It’s just, that might not be the only cell type that matters.
Q - A question a lot of people have had is, once that mRNA gets into our cells and codes for that spike protein, does it just code, does each strand of mRNA just code for one spike protein? And then does the Post-It note, or the mRNA get destroyed or dissolve, or does it code for multiple proteins and last for maybe an hour or a day? Like how long does the mRNA from this vaccine actually last in our cells, approximately?
- A- So the RNA gets read multiple times, so it’ll just keep — it gets read over and over and over again, so that you make a lot of the spike protein, which will then get expressed on the surface of the cell to stimulate the immune system. Average RNAs in your cell will last some time, generally minutes to hours, but some of them will last a day or more, and these, the RNA vaccines are engineered to be stable, and so the information we’ve seen is that they’ll last a couple of days.
Q - So we have the mRNA inside of a lipid nanoparticle, what else goes in in the vaccine obviously it’s got to be some type of saline solution or something?
- A - Right, that’s it. It’s basically just delivered in some, essentially, some, yeah, salt water set to match the saltiness of your own body. So that it’s essentially as “natural” as possible.
Q - It seems like a question that a lot of people have with vaccines in general is, “okay, well what else do they put in them?” And from my understanding with this Pfizer and BioNTech vaccine, they came out and said, “we didn’t put any adjuvants or preservatives in this particular vaccine.” Why are adjuvants used sometimes in vaccines?
- A - So essentially, usually adjuvants are used, and it goes back to what we said about immune memory at the beginning, you know your immune system, some remembers some things really well and remembers other things really poorly, and there are complexities there, but the rule of thumb is that the bigger the threat, then the bigger the memory. It’s a lot like, you know, you might not be able to remember what socks you put on two days ago, but if you’re almost in a car accident at some particular intersection, you’re going to remember that intersection for a very long time, right? Because it was a memorable event, and so vaccines have to deal with the same thing: that the immune system is good at ignoring things that aren’t very threatening, and so adjuvants are a way of providing the immune system a stimulation that says, “hey, this thing that you’re about to see, this is a potential threat, and you should make a substantial immune response to it and remember it,” and so that’s if you just inject a protein by itself. That protein’s inert; it’s non-threatening; it’s not replicating; it’s not going to do anything to you. And so the adjuvant is the immune stimulus to get you going. An RNA vaccine essentially ends up encoding its own stimulation, so it accomplishes that on its own.
Q - The lipid nanoparticle has done its job. It’s brought the mRNA into the cell, and now it’s the ribosome’s job to actually code, or basically essentially build a protein out of that structure?
- A- Right, so what your immune system ends up needing to see in the end are proteins, because that’s what the virus itself is made out of, proteins. The spike proteins are on the surface of the virus, and it’s those proteins that an antibody or T cells would recognize and your cells are making proteins all the time, as instructed by RNA messages. So now instead they’re going to make these viral spike proteins, and that’s what the immune system will start recognizing, and that does get triggered by just the normal protein synthesis machinery in the cells, which are the ribosomes and the amino acids already in your cells.
Q - Why not just skip a step and use a vaccine that uses the spike protein itself? Why go through this extra step of the RNA?
- A- One of the classic ways to make a vaccine is to have the vaccine be the protein, be the viral spike protein or be a viral nanoparticle. And there are vaccines that work fantastically well that way, and some of the original vaccines going back to the early 20th century are that way; that’s the tetanus vaccine and diphtheria vaccine, which are incredibly successful. And in fact, some of the COVID-19 vaccines currently being worked on are protein vaccines, and there’s a reasonable chance those will succeed as vaccines. A downside to protein vaccines is that you have to manufacture the protein, and the manufacturing process for any given protein is its own unique manufacturing problem, and so in terms of just a physical production problem, you’ve got to solve that production problem. And since that’s unique, the FDA has to basically review every step of it and agree that everything is fine about that. And viral proteins tend to be kind of unusual proteins; they’re not super simple to manufacture, so it can take some time and energy to figure out how to solve that, basically, manufacturing problem, that biochemistry protein synthesis problem. The RNA vaccines bypass that problem, because the manufacturing process is always the same. The RNA encodes a different sequence, but molecularly, it’s the same manufacturing process, and so FDA approval and what not is all really fast, because it just it looks the same from a manufacturing standpoint. So that’s why the RNA vaccines have gone through phase one, phase two, phase three trial so fast and gotten FDA approval so quick is because they were they were very fast to manufacture and very fast to approve, because it’s, largely, once they solve the problem once, it’s plug and play.
Q - So along those lines, do you think this is really the future of vaccine development, using this type of technology?
- A - The results are incredibly encouraging, right? This is the first time ever in human history there’s been a vaccine developed within a calendar year, and not only that, now it’s actually been three, right? There have been three successful phase three clinical trials within a single calendar year. That’s never happened for anything. So those are phenomenal successes in the RNA vaccine showing 95 efficacy, right? And fantastic efficacy in the elderly and fantastic efficacy against severe disease. Those are huge wins and RNA vaccines are definitely going to be successful solutions again in the future. They’re likely to still be part of the vaccine toolbox. We don’t think they’ll solve every problem. There are some things that I think they’re good at, and there are other things that other vaccine technologies may be better at. But in terms of speed nothing can match this. You know vaccine development, classically, is frequently a 20-year process, right? Or, you know, let’s say a 10-year process, and instead you’re talking about a 10-month process. You know, it’s not only a 10-month process, but a 10-month process that really involved a huge amount of safety data on all, right? 70,000 doses being given and tested to validate both the efficacy and the safety that clearly RNA vaccines have a very promising future.
Q - From my understanding, mRNA does its work just in the cytoplasm of our cells. Is that correct?
- A- That’s correct. So yeah, I’ve gotten lots of questions about, “well wait, isn’t this genetic engineering? I don’t want to be genetically engineered.” I’m like, well, fair enough, I don’t want to be genetically engineered either, but this is RNA; it’s just messages. They’re transient, temporary, they don’t become part of your body. It’s just not the same thing as DNA.
Q- Now what about, speaking of DNA, the AstraZeneca vaccine candidate that utilizes DNA?
- A- Yeah, so both the AstraZeneca approach and the Johnson & Johnson approach use a viral vector, and it is a viral vector that contains DNA, but really it’s about the virus. So they’re using a different virus and adenovirus as a delivery system into your cells, essentially, sort of like giving you one viral infection to teach your immune system how to fight another viral infection. That’s also transient DNA that doesn’t become part of your DNA, That’s just the virus’s DNA, and those viral vectors they’ve been “gutted,”so that they can’t become another adenovirus. It’s like taking a car and taking out the engine, you know, and even and taking out the seats. It still looks like a car from the outside and you can put some new stuff in it, and you’re sort of showing that to the immune system to teach you what something looks like, but it’s not going to go drive off on its own or anything.
Q - So going back to our kind of step-by-step process, we have the mRNA. The ribosome then codes for a spike protein. Does that spike protein then get released from our cells? Does it get expressed on the surface of our cells or both?
- A- Both. Predominantly, it’s getting expressed on the surface of the cells, and that’s just um, that’s where, well, that’s a good way for it to be shown to the immune system, basically.
Q - So it gets shown to the immune system and then what happens?
- A- A thousand different things. An immune response is a really complicated, orchestrated dance but, essentially, you have in your body right now parts of your adaptive immune system that can potentially recognize any possible virus that would ever exist. But to do that, you have billions of cells that are all really rare, so it’s basically, there’s like one in a million cells somewhere that could actually make the antibodies that would recognize the virus that would stop it. And same thing with the T cells, so what has to happen is those very rare cells have to be exposed to this new protein, and then since those cells are so rare, they’re not very useful when they’re, you know, one in a million, one in a billion cells in your body. So those cells have to grow and divide and multiply until there are millions of them, and that takes time. And that’s one of the big goals of a vaccine is to, really, the whole point of a vaccine is to show your immune system what the virus looks like before you’re infected, so that your immune system can go through that learning process and that growth process on its own, on your immune system’s own time, and get you to a point where now, okay, you’ve got the antibodies, and you’ve got the T cells, and you’re going to have that immune memory all before you ever get exposed to the virus. So normally when you get exposed to the virus, the virus gets the head start. Okay and then your immune system is playing catch-up; your immune system has these rare cells that can potentially protect you, but they’re rare, and they have to grow from one cell into a million cells, and usually that takes a week, and you get sick for that week in the meantime.
Q - So you talked about this cascade of immune system effects and response to either a vaccine or a natural infection with a vaccine. What symptoms would you expect when the immune system is really ramping up and responding?
- A- These vaccines are safe; that doesn’t mean they’re not gonna make it not feel so great for a day or two or have a little bit of a fever, and that can be a really positive thing, because essentially this goes back to your immune system is really designed to remember things that were something of a threat, you know, and so it’s, uh, you really do kind of have to earn your immunity some, a lot like going to the gym and working out. You know, if you get really sore, that can really be a positive sign. Same kind of thing for a vaccine; if you’ve got some swelling, if you’ve got some redness, if you got a little bit of a fever, those are basically all straightforward signs that your immune system is working, is doing its job of recognizing that vaccine and building the tools and weapons to fight the virus if they see it, and usually for most vaccines, that can go on for, you know, one day, two days, three days, and that’s been what people have been seeing with these RNA vaccines as well. Most people get a bit of redness and a bit of soreness, and some people get a real fever for a day, and that’s honestly just a positive sign that your immune system is fighting it.
Q- So, I mean, “side effect” is almost the wrong terminology for that. I mean, it’s really kind of an expected immunogenic response ?
- A- Exactly, and that’s why it’s important to recognize that safety is really important for vaccines, because vaccines are given to healthy people, and that’s always been a key feature of vaccines is paying a lot of attention to safety, but that’s different than getting sore or feeling a bit tired. Those can be sort of, essentially, “on target” effects, signs that you’re immune, signs that the vaccine is really working.
Q- The guidance from the FDA and the CDC with the the Pfizer-BioNTech vaccine is that even people who have had a previous SARS-CoV-2 infection should get the vaccine ? I think a lot of people were initially confused by this. Why do you think they made that recommendation?
- A- It’s because we don’t, like as of today and certainly as of a couple weeks ago, we don’t have a good grasp of how long does protective immunity last after you’ve had COVID-19? And we also don’t know how long it lasts after the vaccines. But, so far that the vaccines are looking good. In our data, when we looked at immune memory in people, right, we were seeing something like 95 percent of people had what we consider immune memory. That looks good, but that still doesn’t prove that those people are going to have protective immunity. Really, you have to have bigger, longer studies to wait and see, you know. How long are people protected? And so, we think the vaccine recommendation is the right one. If we knew for sure that catching COVID-19 really did give you protective immunity for a long time, then we think the vaccine recommendation would be “no, don’t bother.” But as it stands right now, we don’t know that, and so it makes sense to still recommend getting the vaccine.
Q - And is it possible that the vaccine can actually give longer immunity than a natural infection?
- A - It’s possible. There are definitely vaccines that do that. So the papillomavirus vaccine is a fantastic example of a vaccine where the vaccine works way better than natural infection at generating protective immunity and long lasting immunity. The opposite also occurs. I mean, the normal flu vaccine really gives pretty short-lived immunity, but if you actually catch the flu, your immunity to that flu is really quite long-lasting. So it can go both ways, and since RNA vaccines are new, we don’t have a historical reference point for comparison. So far the data with the RNA vaccines has been fantastic, and really the big unanswered question with them at this point is durability. How long are they going to last? And right now, we don’t know how long durability is going to last for the vaccine compared to having had the infection.
Q — The Pfizer-BioNTech vaccine and the Moderna vaccines are very similar. Why does the Pfizer vaccine need to be stored at negative 94 degrees fahrenheit, when the Moderna vaccine just needs regular refrigeration?
- A- It’s pretty cold, right? Well the Moderna, one requires the very cold for long-term storage but for a shorter term, it can do better. And in fact, there are other RNA vaccine formulations that have been published later in 2020 that could actually do room temperature storage. It comes down to the nature, the precise nature, of those lipid nanoparticles, and how stable they are.
Q- If you had a family member or a close friend say to you, “You know, you’ve studied vaccines in immunity your entire career. This vaccine looks promising, but it, you know as you mentioned, the timeline has been so much shorter than what we’re used to with vaccines and it’s using a new technology, this rna technology. Should I be nervous about this?” What would you say?
- A- The answer is no, don’t be nervous. Definitely get vaccinated. If you can get vaccinated, I mean obviously for one right now, the COVID-19 threat in the population is horrible, right? I mean, we’ve crossed thresholds of like 3,000 deaths a day in the country. I mean, those are, uh, it’s a really bad situation, and on the flip side, these vaccines are, you know, 95 percent effective. That, in two totally independent trials of huge numbers of people, that data is really strong. These vaccines definitely work, and yeah I certainly get questions about safety, which are reasonable questions to ask, again, because you said, because of the speed. And so there are two parts of it: one is you would be really hard-pressed to find any medicine that has had this much safety data already by the time it becomes publicly available. Again, 70,000 people have already gotten the vaccine and been tracked for safety. That’s a huge amount of safety data, way more than most medicines get when they come to market. So those are — and the reason for accumulating all that was actually because of speed. That’s actually to find results quick enough, they had to have a huge number of people involved in the study, and so as a result, they got a ton of safety data, and they’ve also got safety data going, you know, for essentially six months from the earlier clinical trials that got started in the summer. Really the best way to think about the speed of development is one: this is a technology that could move very fast through manufacturing and that’s really where a lot of the speed came from was manufacturing. The safety parts of it is the same amount of time as it basically always takes. And the other thing that’s been fast about it has been problems that money could solve. So normally for developing a vaccine, somebody goes through a phase one trial and then waits and then goes through a phase trial and waits and then goes to a phase three. They don’t invest a huge amount of money up front, because there’s a good chance that they would lose that money, and instead in this situation, right, going back to March, companies governments, and non-government organizations were all saying, “okay, invest the billion dollars up front, you know, and sure, we may lose that money, but if it works we’ll have a vaccine, you know, a year faster than we otherwise would, because we’re just paying for the manufacturing to get going up front.” That’s just the problem money can solve. You could just be losing that money in the end, but you’re not taking any shortcuts. You’re just starting the process a lot earlier than you would otherwise and, sure enough, things worked out incredibly well, right, and these vaccines are actually working, and so now there are already vaccine doses being delivered instead of the companies now starting to manufacture them and then being delivered, you know, six months or more later.
Q- And as you mentioned, there’s good data and there’s a lot of data about safety in the short term. What about long-term potential side effects? I know that’s another concern.
- A- Yeah, that’s a good question, and that was one of the main ones that the FDA wanted to consider as well, and so basically they did a review of vaccine literature and said ,”yeah in the past for all these other vaccines, any important vaccine safety signature was clear within two months,” and so that’s why the FDA specifically demanded that there be two full months of safety data on these large trials and that’s what’s being reviewed by the FDA, and they’ve looked fine.
Q- So in other words, if, based on on the extensive history we have with vaccines, if you don’t see a safety concern in the first two months of use of the vaccine, it’s unlikely to see long-term side effects down the road?
- A- Right, yeah, that’s exactly right. We’re one of the best places in the world studying the immune system, and we can actually look at all these different immune responses to COVID-19 at the same time, which most places can’t. So we’re continuing to examine that both to try and understand acute disease, you know why people end up in the hospital, as well as immune memory to this virus, and it’s, uh yeah, it’s a lot of work, but it’s important. So those are the problems we keep trying to solve.
We can make mutation-beating coronavirus vaccine in six weeks
- It was “highly likely” that all vaccines against the coronavirus works against the mutated strain detected in Britain, but companies could also adapt the vaccine if necessary in six weeks.
- “Scientifically, it is highly likely that the immune response by this vaccine also can deal with the new virus variant,”.
- But if needed, “in principle the beauty of the messenger technology is that we can directly start to engineer a vaccine which completely mimics this new mutation — we could be able to provide a new vaccine technically within six weeks.”
- The variant detected in Britain has nine mutations, rather than just one as is usually common.
- Nevertheless, the vaccine developed with Pfizer would be efficient because it “contains more than 1,000 amino acids, and only nine of them have changed, so that means 99 percent of the protein is still the same”.
- Tests are being run on the variant, with results expected in two weeks.
- We have scientific confidence that the vaccine might protect but we will only know it if the experiment is done… we will publish the data as soon as possible.
