COVID-19 : JV / Acquisition Of Next-Gen Vaccine Platforms.
Feeling a little overwhelmed by all the vaccine news on the different COVID-19 vaccines? You are not alone: As per WHO Data 140 vaccines are in preclinical trials, about 30 are in human trials, and 2 have been approved, 1 in China that will only be given to members of their military and 1 in Russia. Neither of these has undergone the testing needed to assure a safe, effective vaccine. Visit this site which is tracking/updating all the COVID-19 vaccines in trials here.
Top 5 : What are the Top 5 most promising COVID-19 vaccine candidates?
- With the strong Phase 1/2 immune response results on 20 July, subsequent stoping of trials and now resumption of trials, Oxford University’s vaccine jumps to no. 1 in this list — leading the pack into Phase 3 trials in the UK, US, Brazil and South Africa
- Moderna drops to no. 2 with its unproven mRNA platform, even following its successful Phase 1 trial results
- China’s CanSino adenovirus vaccine also saw data published on 20 July — showing a rapid immune response and no serious adverse reactions
- Maybe the safest bet is to use the long-proven route of an inactivated virus vaccine — if so, the Chinese company Sinovac is the one to watch.
- Novavax got the biggest sum so far from Operation Warp Speed — $1.6 billion, announced 7 July — but has yet to get to Phase 3.
Consensus among experts is that only an effective COVID-19 vaccine will end the pandemic. This Comment focuses on how this pandemic has accelerated the development of vaccine platforms distinct from classical vaccines; these novel platforms may also increase the response time when new viruses emerge in the future.
An outbreak of highly pathogenic avian influenza (HPAI) virus of the H5N1 subtype was diagnosed in Hong Kong in 1997, with 18 human cases including six deaths. This was the first known outbreak of influenza A virus resulting from direct transmission of an avian influenza virus from chickens to humans without an intermediate reservoir such as pigs. This outbreak increased the awareness of the risk of a devastating pandemic, and showed that more resources should be diverted to pandemic preparedness planning. Many considered this an overblown response to a small outbreak, aimed at acquiring more research funding, especially when years went by without an H5N1 pandemic, despite the widespread, continued circulation of HPAI H5N1 in poultry with regular spillover to humans, resulting in more than 850 human cases and over 450 deaths to date. The emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002 put everyone on alert for a while, but this pandemic was contained through old-fashioned contact tracing and isolation procedures after causing >8,000 cases in 27 countries. Subsequently, the 2009 H1N1 pandemic turned out to be relatively mild, with a low case fatality rate. The Ebola virus epidemic in West Africa in 2013–2016 was a turning point. Whereas the largest previous Ebola virus outbreaks had resulted in several hundred cases in confined areas, this epidemic resulted in close to 30,000 cases in ten countries and took more than three years to bring under control. The unprecedented number of cases, geographic spread, and enormous amount of money and effort needed to end this epidemic made clear that zoonotic viruses pose an immense threat to global health and economies. Efforts were launched to identify viruses with epidemic potential, and money was invested in developing vaccines against some of these, as well as in the development of new, rapid vaccine platforms. Now, six months after the discovery of SARS-CoV-2, antivirals and vaccines are in development, with many treatment options and vaccines in clinical trials worldwide. Even though antivirals are important to dampen the disease burden of the current pandemic, effective vaccines are essential to control it. The World Health Organization (WHO) estimates that there are 133 COVID-19 vaccines in development. Many of these are novel platforms with little pre-existing data on safety and efficacy in humans.
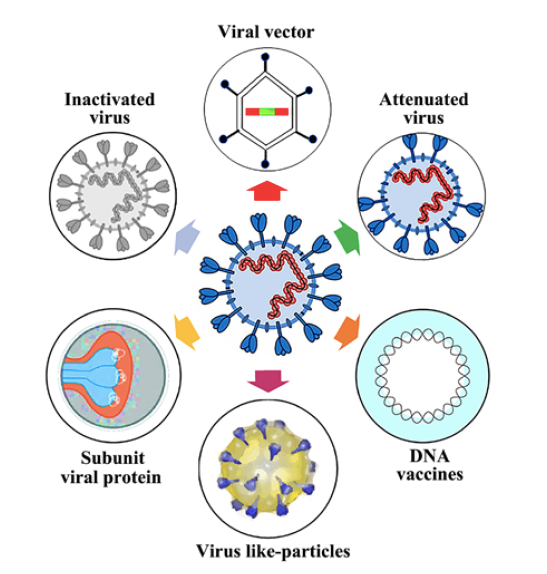
Lets simplify it and talk about the advantages and disadvantages of the 8 different vaccine development platforms being used.
Existing development platforms
Four of the 8 platforms are currently used to make vaccines we use every day. Those 4, however, are behind in the development timeline as compared to the COVID-19 vaccines being researched using 4 next-generation vaccine platforms. Key advantages to the 4 original models are that existing manufacturing capacity can be used and that, if produced, the final product will be generally a more temperature-stable one.
The current threat of avian influenza to the human population, the potential for the reemergence of severe acute respiratory syndrome (SARS)-associated coronavirus, and the identification of multiple novel respiratory viruses underline the necessity for the development of therapeutic and preventive strategies to combat viral infection. Vaccine development is a key component in the prevention of widespread viral infection and in the reduction of morbidity and mortality associated with many viral infections. In this part, coronavirus vaccine, especially SARS-CoV vaccines are mainly discussed.
Coronavirus vaccines can be inactivated coronavirus, live attenuated coronavirus, or S protein-based. Besides, there are still vectored vaccines, DNA vaccines, and combination vaccines against coronaviruses. Vaccines targeting several animal CoVs have been developed, and some have been demonstrated to be efficacious in preventing viral infection. However, a phenomenon of enhanced disease following vaccination has been observed in cats upon infection with feline infectious peritonitis virus following previous infection, vaccination, or passive transfer of antibody. The phenomenon is not fully understood but is believed to be a result of enhanced uptake and spread of the virus through binding of virus-antibody immune complexes to Fc receptors on the surfaces of macrophages; low-titer (subneutralizing) antibodies directed against the S protein are mainly responsible. Although antibody enhancement appears to be limited to feline infectious peritonitis virus among CoVs, similar concerns have been raised with regard to SARS-CoV. Previously infected mice and hamsters are protected from subsequent infection with SARS-CoV in the absence of enhanced disease, and vaccine studies and passive immunoprophylaxis performed with mice and hamsters suggest that previous exposure and the presence of NAbs provide protection.
The 4 existing development models that have COVID-19 vaccine candidates being researched:
- Whole cell inactive eg, polio shot
- Live attenuated eg, MMR vaccine
- Protein subunit eg, flu vaccine
- VLP (virus like particle) eg, Gardasil
A concern with the live attenuated vaccine would be reversion through mutation to the wild virus that is rarely seen with our live polio vaccine.
Novel development platforms
The 4 novel, or “next generation” vaccine platforms are ahead in the race since all are based on the viral genetic sequence of the SARS-CoV2 virus which was discovered very early in the pandemic. In general, these vaccines may be easier to make and don’t need live virus in the production process, but will be more temperature-unstable, so shipping and storage will need to be carefully monitored to avoid vaccine inactivation before administration.
The 4 next generation development models that have COVID-19 vaccine candidates being researched:
- Viral vector eg, Ebola vaccine
- DNA-based None licensed
- RNA-based None licensed
- Antigen-presenting cells None licensed
All vaccine development involves finding an antigen(s) that is recognizable by the immune system, leads to the production of antibodies that work to prevent infection, and is not so reactogenic as to preclude its use. Ideally, memory T and B cells will be stimulated resulting in long term immunity.
Different antigens produce different levels of antibodies and can trigger different types of immune response (T cell, B cell, and cell-mediated). So:
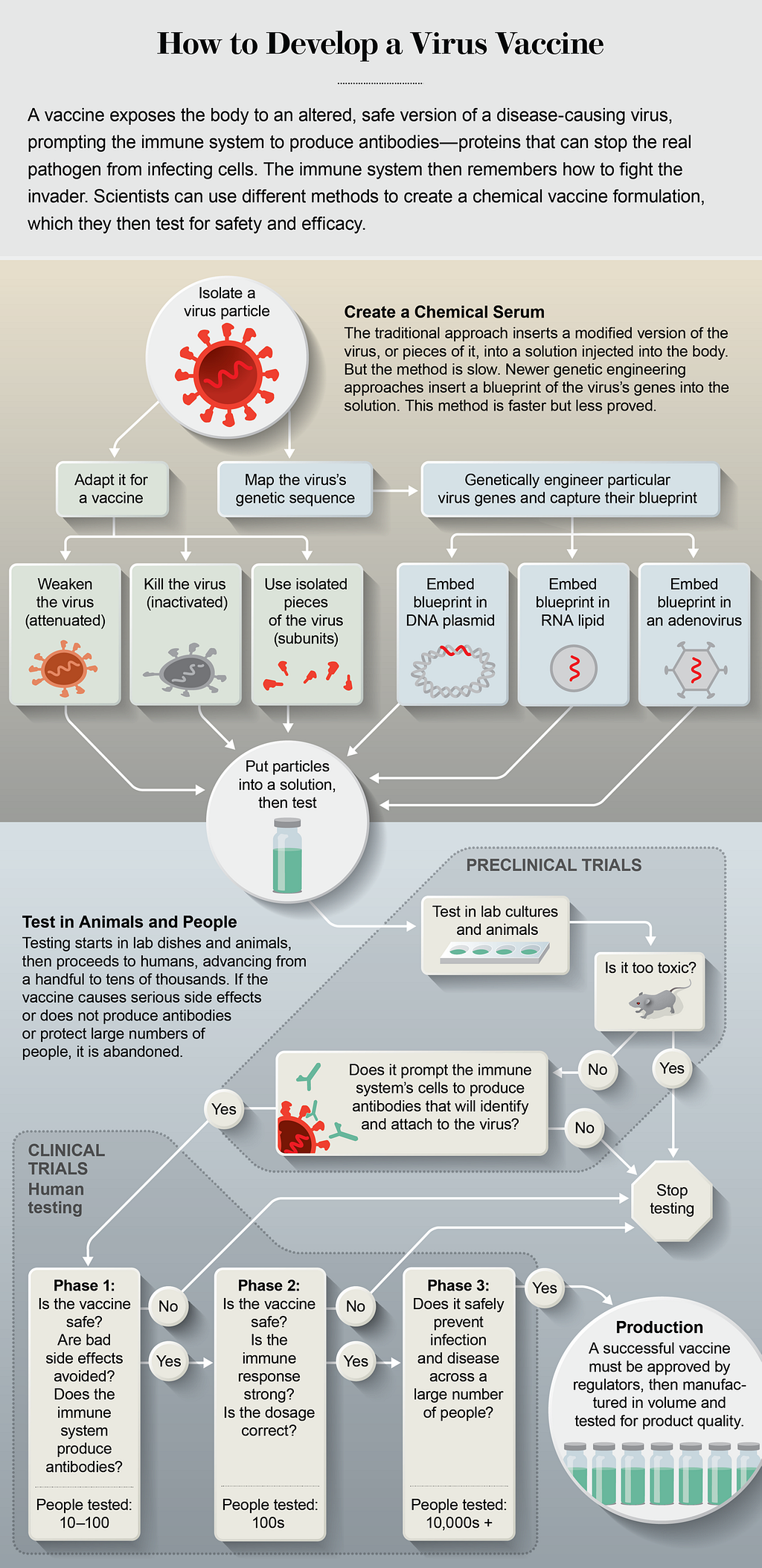
Step 1: Find the right antigen. Step 2: Get the antigen to be “seen” by the human immune system
Classic vaccine platforms
The vast majority of vaccines currently licensed for human use can be divided into virus-based or protein-based vaccines (Fig. 1). The virus-based vaccines can consist of inactivated virus that is no longer infectious, or live-attenuated virus. Since whole-inactivated viruses do not replicate, adjuvants are required to stimulate the immune system. Live-attenuated virus vaccines are classically generated by passaging in cell culture until it loses its pathogenic properties and causes only a mild infection upon injection. Protein-based vaccines can consist of a protein purified from the virus or virus-infected cells, recombinant protein or virus-like particles. Virus-like particles consist of the structural viral proteins necessary to form a virus particle, but lack the viral genome and non-structural proteins. Protein-based vaccines require the addition of an adjuvant to induce a strong immune response. Two COVID-19 vaccines based on these classical platforms are currently in clinical trials, one based on whole-inactivated virus and one consisting of recombinant protein (Fig. 1).
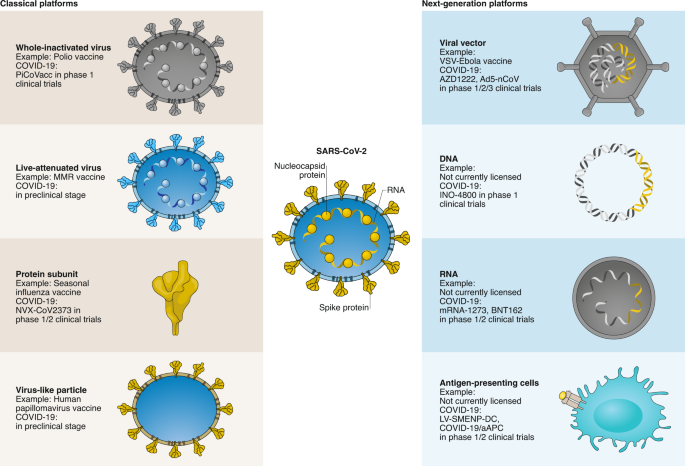
These classical vaccine platforms have contributed to major public health breakthroughs, such as the eradication of smallpox and a vaccine to prevent cancer. However, certain limitations are associated with several of these platforms that make them less amenable to fast vaccine production in a pandemic. In the case of SARS-CoV-2, large quantities of virus would need to be grown under biosafety level 3 (BSL3) conditions for a whole-inactivated vaccine; extensive safety testing is required to ensure live-attenuated viruses are safe and do not easily revert to wild type, and several recombinant proteins need to be produced simultaneously for virus-like particle vaccines.
Inactivated pathogen
The immunogenicity and efficacy of inactivated SARS-CoV vaccines have been established in experimental animals, and one such vaccine is being evaluated in a clinical trial. However, the development of inactivated vaccines requires the propagation of high titers of infectious virus, which in the case of SARS-CoV requires biosafety level 3-enhanced precautions and is a safety concern for production. Additionally, incomplete inactivation of the vaccine virus presents a potential public health threat. Production workers are at risk for infection during handling of concentrated live SARS-CoV, incomplete virus inactivation may cause SARS outbreaks among the vaccinated populations, and some viral proteins may induce harmful immune or inflammatory responses, even causing SARS-like diseases.
Protein immunogens are a different, and traditional, category of anticipated COVID-19 vaccines. Protein immunogens may be delivered as viral subunit proteins, viruslike particles, or as inactivated virus; are produced in a laboratory; and are often administered with an adjuvant to increase response magnitude and durability. The hepatitis A vaccine and the Salk inactivated polio vaccine are examples of licensed inactivated whole-virus vaccines.
How does it work?
The most traditional vaccine approach — one utilized over many decades — is to inject someone with the inactivated virus. This stimulates the immune system to produce antibodies, while the virus is either killed before injection or weakened sufficiently so that it cannot cause a serious infection. Inactivated viruses are used against influenza, for example, and in the global effort to eradicate polio.
Who is doing it?
Here once again the Chinese are in the lead. The Chinese company Sinovac, in partnership with a number of leading medical research institutes in China, designed a vaccine by isolating SARS-CoV-2 samples from infected hospital patients and growing the virus in cell lines before inactivating it with a chemical agent. It was formerly called PiCoVacc (for “purified inactivated SARS-CoV-2 vaccine”) but has now been more simply renamed CoronaVac.
An international team has a different approach, using a vaccine that is already widely deployed: the BCG vaccine against tuberculosis. It has been shown to protect against other respiratory diseases, too, so researchers are hoping it might be effective against COVID. On 9 June a paper in PNAS seemed to suggest that BCG vaccines given earlier in life were reducing death rates in some populations. (BCG is an inactivated bacterial pathogen, not a virus.)
What’s the latest?
The Chinese team has made impressive progress with its inactivated viral COVID vaccine. In a paper published in Science on May 6, the team reported that their candidate vaccine had “induced SARS-CoV-2-specific neutralizing antibodies in mice, rats and non-human primates.” It also “provided partial or complete protection in macaques” against deliberate infection with the virus. On June 13 Sinovac posted initial results for a Phase 1/2 trial involving several hundred people: 90% of the volunteers tested positive for protective antibodies. The company inked a deal on 11 June to push on to Phase 3 trials in Brazil, where the disease is still running rampant. It will simultaneously construct a vaccine manufacturing plant capable of producing 100 million doses annually.
Because BCG already has a decades-long history of safe use as a vaccine, trials to see whether it is effective against COVID have gone straight to Phase 3. Trials are currently underway among 10,000 frontline health workers in Australia, run by Murdoch Children’s Research Institute, and in the Netherlands among a further 1,500 health workers.
Any drawbacks?
Growing large volumes of viruses to use in vaccines is a long and arduous process, so the traditional approach will be the slowest to scale up globally. Believe it or not, most attenuated virus vaccines are made using huge numbers of chicken eggs.
Live Attenuated
- To date, live attenuated vaccines for SARS-CoV have not been evaluated.
- However, systems have been developed to generate cDNAs encoding the genomes of CoVs, including SARS-CoV.
- The panel of cDNAs spanning the entire CoV genome can be systematically and directionally assembled by in vitro ligation into a genome-length cDNA from which recombinant virus can be rescued.
- This system has been used for genetic analysis of SARS-CoV protein functions and will enable researchers to engineer specific attenuating mutations or modifications into the genome of the virus to develop live attenuated vaccines.
- While live attenuated vaccines targeting respiratory viruses, including influenza viruses and adenoviruses, have been approved for use in humans, the observation that infectious virus is shed in the feces of SARS-CoV-infected individuals raises concerns that a live attenuated SARS-CoV vaccine strain may also be shed in feces, with potential to spread to unvaccinated individuals.
- Another concern is the risk of recombination of a live attenuated vaccine virus with wild-type CoV; however, there may be ways to engineer the genome of the vaccine virus to minimize this risk.
- Codagenix has successfully synthesized a live-attenuated vaccine candidate for COVID-19 called CDX-005.
- The vaccine candidate is undergoing safety and efficacy studies in animals, according to the firm, which has got preclinical data in early July. sINCE THE preclinical trials are complete, CDX-005 will being tested in a phase I trial in the fall.
- CDX-005 is one of the few vaccine candidates currently under development using the live-attenuated approach, which mimics SARS-CoV-2.
- The company is collaborating with the Serum Institute of India to develop CDX-005 and the phase I clinical trial in the fall has begun. The compound grows rapidly in cell culture, which means that it can be produced quickly.
Protein Subunit
- The roles of S protein in receptor binding and membrane fusion indicate that vaccines based on the S protein could induce antibodies to block virus binding and fusion or neutralize virus infection.
- Among all structural proteins of SARS-CoV, S protein is the main antigenic component that is responsible for inducing host immune responses, neutralizing antibodies and/or protective immunity against virus infection.
- S protein has therefore been selected as an important target for vaccine and anti-viral development.
- Although full-length S protein-based SARS vaccines can induce neutralizing antibody responses against SARS-CoV infection, they may also induce harmful immune responses that cause liver damage of the vaccinated animals or enhanced infection after challenge with homologous SARS-CoV, raising concerns about the safety and ultimate protective efficacy of vaccines that contain the full-length SARS-CoV S protein.
Viral proteins
How does it work?
This is another traditional method for vaccinations: genes that code for proteins from the pathogen — in COVID’s case, mostly the notorious spike protein — are spliced into different viruses, which are then mass-produced. The approach has been used successfully in the HPV vaccine, for example. Virus-like particles can also be produced in plants.
Who is doing it?
Sanofi Pasteur, the vaccines division of Sanofi, is repurposing its earlier SARS vaccine efforts into COVID. Its recombinant DNA approach in cell lines has already been licensed to produce an influenza vaccine, distributed since 2017 in the US under the brand FluBlok. This should produce a quicker and more stable product than vaccines traditionally produced in chicken eggs.
This approach is also being used by a team at the University of Pittsburgh, whose members had already worked on SARS and MERS and quickly repurposed their spike protein vaccine to target SARS-CoV-2. Its purified protein can be delivered in a “microneedle array,” a fingertip-sized patch of 400 tiny soluble needles that affixes to the skin like a Band-Aid.
Separately, Novavax has developed a way to package SARS-CoV-2’s spike proteins into nanoparticles that should enhance the immune response by better mimicking the virus. In Canada, Medicago began producing virus-like particles of the coronavirus — expressed in leaves of Nicotiana benthamiana, a wild relative of tobacco — just 20 days after the viral genome was published.
What’s the latest?
Sanofi’s hare-and-tortoise approach may soon begin paying dividends. The company was a slow starter, but has brought forward Phase 1/2 trials from December to September. “We are the only vaccine in the race that’s off a proven platform that works in scale,” said its CEO. On 24 June Sanofi said Phase 3 trials could begin by December, and that it expects to have 100 million doses of its vaccine prepared by the end of the year — even before it has been proven to work. If all goes to plan, another 1 billion doses can be manufactured in 2021.
The Pittsburgh team won the race to produce the first peer-reviewed paper on a COVID vaccine trial, reporting in mid-March that its microneedle vaccine had “elicited potent antigen-specific antibody responses” when tested in mice. However, Phase 1 human trials have not yet begun, and the scientists warn that getting results “would typically require at least a year and probably longer.”
Novavax began Phase 1 trials on May 26 in Australia in 131 human volunteers, with results expected later in July. The company is developing scaled-up production that could potentially deliver 100 million vaccine doses by the end of 2020, and 1 billion doses starting in 2021. It secured the biggest payout so far from the US Operation Warp Speed, with $1.6 billion announced on 7 July. The vaccines — 50 million doses for starters — are expected to be delivered to the US government in February 2021.
Medicago announced positive results for a trial of its COVID candidate vaccine in mice on May 14, and aims to start human trials in the summer. It can already produce 120 million doses of the vaccine per year in its current facilities, and aims to scale up to 1 billion per year by 2023.
Any drawbacks?
As with growing viruses directly, growing large amounts of viral proteins takes time. Cell lines may be quicker than chicken eggs but scaling up to the billions of doses will take a lot longer than the mRNA/DNA approach.
Next-generation vaccine platforms
The main advantage of next-generation vaccines is that they can be developed based on sequence information alone. If the viral protein(s) important to provide protection from infection or disease, and thus for inclusion in a vaccine (that is, the vaccine antigen), is known the availability of coding sequences for this viral protein(s) suffices to start vaccine development, rather than having to depend on the ability to culture the virus. This makes these platforms highly adaptable and speeds up vaccine development considerably, as is clear from the fact that the majority of COVID-19 vaccine clinical trials currently ongoing involve a next-generation platform (Fig. 1).
Viral vector platform
For COVID-19, several viral vector, nucleic acid-based vaccines and antigen-presenting cells are in (pre)clinical development (Fig. 1). Viral vector vaccines consist of a recombinant virus (that is, the viral vector), often attenuated to reduce its pathogenicity, in which genes encoding viral antigen(s) have been cloned using recombinant DNA techniques. Vector vaccines can either be replicating or non-replicating. Replicating vector vaccines infect cells in which the vaccine antigen is produced as well as more infectious viral vectors able to infect new cells that will then also produce the vaccine antigen. Non-replicating vector vaccines initially enter cells and produce the vaccine antigen, but no new virus particles are formed. Because viral vector vaccines result in endogenous antigen production, both humoral and cellular immune responses are stimulated. One advantage of these viral vector-based vaccines is therefore that a single dose can be sufficient for protection, as in the case of the vesicular-stomatitis virus-based Ervebo vaccine against Ebola virus.
The viral vector approach takes an infectious human virus, adenovirus is one of several being used, and genetically modifies it to produce the COVID-19 antigen and perhaps also to attenuate the viral vector. The carrier virus infects human cells, injects its genome containing the gene coding for the COVID-19 antigen, and the human cell makes the antigen.
The vector can be replicating, meaning new viral carrier particles (essentially, new vaccine) are produced that spread to new cells. Non-replicating viral vectors also are in development. Viral vector vaccines seem to be very immunogenic and most likely only one dose will be required, unlike most of the others for which 2 doses will be needed.
Drawbacks with the viral vector vaccine include the possibility of side effects and that someone with a recent infection with a virus in the vector family may have an immune response that destroys the viral vector before it can infect enough cells.
That said, viral vector vaccine trials have a good track record with other emerging viruses, Ebola being one.
Several groups have reported preclinical evaluation of vaccines utilizing other viruses as vectors for SARS-CoV proteins, including a chimeric parainfluenza virus, MVA, rabies virus, vesicular stomatitis virus (VSV), and adenovirus. Chimeric bovine/human parainfluenza virus 3 (BHPIV3), a live attenuated parainfluenza virus vaccine candidate, was utilized as a vector for the SARS-CoV structural proteins including S, N, matrix (M), and envelope (E), alone or in combination. Studies with vectored vaccines further demonstrate that induction of S protein-specific NAbs is sufficient to confer protection.
Adenovirus vaccine
How does it work?
Adenoviruses, which exist in the wild in humans and typically cause mild infections such as the common cold, have been genetically engineered to express viral antigens found in SARS-CoV-2, usually those of the infamous spike protein that the coronavirus uses to break into human cells. These engineered adenoviruses, when put into a vaccine, trigger an immune response in the human body, protecting against COVID-19.
This is a new technology: no adenovirus vector vaccines for other diseases are yet widely available, though vaccines for HIV, influenza, Ebola and malaria using this platform are in clinical trials and an Ebola vaccine has been briefly deployed.
Who is doing it?
Probably the highest-profile effort is the ChAdOx1 nCoV-19 vaccine candidate from Oxford University’s Jenner Institute. (ChAdOx1 stands for “chimpanzee adenovirus Oxford 1.”) The Chinese company CanSino Biologics — the medical science arm of the People’s Liberation Army, no less — has completed Phase 1 trials with an adenovirus vector vaccine called Ad5-nCoV.
A big-name corporate player is Johnson & Johnson, via its subsidiary Janssen, which uses a genetically modified human adenovirus technology it calls AdVac. This is a proven platform, which was used to produce thousands of doses of company’s Ebola vaccine deployed in the Congo in November 2019.
Adenoviruses are not the only viral vectors that can be used: pharmaceutical giant Merck says it is working on a potential COVID vaccine using an engineered vesicular stomatis virus, previously used successfully in its Ebola vaccine. Another collaboration Merck is involved in uses an attenuated live measles vaccine.
What’s the latest?
CanSino reported Phase 2 results in the Lancet on 20 July. According to the authors, these showed that the adenovirus vector vaccine “is safe, and induced significant immune responses in the majority of recipients after a single immunisation”. According to Reuters, the company is in talks with Russia, Brazil, Chile and Saudi Arabia to launch Phase 3 trials.
The Oxford team published results on 20 July in the Lancet. “We are seeing very good immune responses, not just on neutralizing antibodies but of T-cells as well,” Adrian Hill, head of Oxford’s Jenner Institute, told Bloomberg. “We’re stimulating both arms of the immune system.” Oxford’s vaccine leads the global race, and could receive emergency use authorization as early as October. Phase 3 trials are now planned, involving tens of thousands of participants in the UK, US, Brazil and South Africa. These will establish whether the vaccine actually protects against the virus in the real world.
Oxford University has partnered with the global pharmaceutical company AstraZeneca for manufacturing purposes. On May 21 it announced an agreement to produce 400 million doses and claims to be able to manufacture 1 billion doses with current facilities. The US government is also betting big on Oxford: its Biomedical Advanced Research and Development Authority (BARDA) put $1billion behind the Oxford/AstraZeneca effort.
Johnson & Johnson, while it has the corporate muscle to produce vaccine doses in large quantities, doesn’t expect to start Phase 1 trials until September, which it says could possibly “allow vaccine availability for emergency use in early 2021.”
Any drawbacks?
Live viruses, even if attenuated, can be risky in immunocompromised people. CanSino’s adenovirus is a human virus, while Oxford uses one from chimpanzees — the latter might be more effective as no-one yet has an immune response to it.
DNA- and RNA-based platforms
Nucleic acid-based vaccines can consist of DNA or mRNA and can be adapted quickly when new viruses emerge, which is why these were among the very first COVID-19 vaccines to enter clinical trials. DNA vaccines consist of a synthetic DNA construct encoding the vaccine antigen. For efficient uptake of the construct into cells, injection needs to be followed by electroporation. After uptake into cells, the vaccine antigen is expressed from the DNA construct. mRNA-based vaccines work on the same principle as DNA vaccines, except that the first steps (nuclear translocation of the DNA construct and transcription into mRNA) are bypassed. Self-replicating RNA vaccines are likely to induce protective immunity using a lower dose, because more vaccine antigen is expressed per cell. Since mRNA is not very stable, these constructs include modified nucleosides to prevent degradation. A carrier molecule is necessary to enable entry of the mRNA into cells; lipid nanoparticles are most commonly used. Nucleic acid-based vaccines induce a humoral and cellular immune response, but multiple doses are required.
The DNA- and RNA-based vaccines need to get those snippets of viral genome that code for a COVID-19 antigen into human cells so they can make the viral antigen that stimulates the immune response. An American company working on a DNA-based vaccine uses a hand-held electrical device to drive plasmids containing the COVID-19 DNA (but just the bit that codes for a spike protein, so no risk of infection) into human cells. The electrical jolt does add some pain in addition to the shot. RNA-based vaccines use a lipid nanoparticle that is taken up by cells. The mRNA vaccines seem to produce more antigen per cell but are much more temperature unstable as compared to DNA vaccines.
The general idea of delivering a synthetic gene is that you need to get it into the area of a cell that will cause that gene to be made into a protein. That occurs in two steps: Transcription, whereby DNA is converted into RNA, and translation, where RNA is converted into protein. Transcription happens inside the nucleus of a cell, whereas translation happens in the cytoplasm. Because the nucleus exists to protect the DNA, it’s a highly guarded region, making it difficult to penetrate. By contrast, the cytoplasm is much easier to penetrate, hence the interest in mRNA.
You can get a much higher rate of delivery with a lesser amount of material. In other words, if the ratio of dosage to actual delivery into cells is 10% for DNA vaccine, it’s more like 20% for an mRNA vaccine, meaning an mRNA vaccine would have lower dosing requirements and also be faster to manufacture in large quantities. The mRNA approach also cuts down the development time.
RNA vaccine
How does it work?
While conventional vaccines work by presenting the body’s immune system with the inactivated real virus or antigens derived from it, injecting mRNA into cells means that they produce the required viral proteins directly inside the human body. mRNA (the “m” stands for messenger) is the molecule that takes instructions from DNA to the cell’s protein factories (called ribosomes). A big advantage of mRNA vaccines is that scientists can skip the laboratory production of proteins by directly injecting the molecular instructions to make the protein into the human body itself.
In this case the RNA sequence is taken from the SARS-CoV-2 virus genome, stimulating an immune response that should later stop the COVID-19 disease. One advantage to mRNA vaccines is a cheaper, faster production process, making them potentially the most scalable to tackle a global pandemic.
The design of the RNA vaccines is more uncertain. Most of the delivery platforms have been stated or are likely to be lipid nanoparticles (LNPs). However, BioNTech has three different lipid delivery platforms (lipoplexes, LNPs and polyplexes) and has not stated which one they are using. The delivery platform that Arcturus Therapeutics will use, the LUNAR system of Synthetic Genomics, is supposed to be widely applicable for multiple inoculation routes and target tissues. However, few developers have stated their inoculation route or the tissue they are targeting their LNPs . Two developers (Arcturus Therapeutics, Imperial College London) are using self-replicating mRNA , two (Translate Bio, Curevac) are using unmodified optimized mRNA sequences , and one (BioNTech) is currently still testing its three different RNA formats. Many companies are likely to use the major structural protein (spike) as the gene of choice, but not all developers have explicitly said so .
Who is doing it?
Moderna — a biotech startup now worth tens of billions, though it has yet to sell a single product — is in the lead. Other teams pursuing the mRNA approach include one based at Imperial College, London; the German-based company BioNTech, which is working in alliance with the drugs giant Pfizer; and CureVac, another German-based company. A Chinese consortium from Fudan University, Shanghai JiaoTong University and RNACure Biopharma is employing a second strategy of using mRNA to create “virus-like particles” in the body to activate an immune response.
What’s the latest?
Moderna’s vaccine (mRNA-1273) was the first to be injected into human volunteers, way back in mid-March. On 14 July it published the results of this Phase 1 trial in a peer-reviewed paper in the NEJM. The news was good: its “mRNA-1273 vaccine induced anti-SARS-CoV-2 immune responses in all [45] participants, and no trial-limiting safety concerns were identified”. Phase 2 trials in 600 participants are underway now, and the company has announced that its 30,000-participant Phase 3 trial will begin on 27 July in the US. Moderna “remains on track to be able to deliver approximately 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021”, it says.
CureVac announced “positive pre-clinical results” for its lead COVID vaccine candidate on May 14 and received approval to start a Phase 1 clinical trial on June 17 in Germany and Belgium. More exciting, perhaps, is the reported involvement of tech superstar Elon Musk, who recently revealed that his company Tesla will soon be “building RNA microfactories for CureVac & possibly others”. Synthetic RNA (and DNA) has amazing potential. This basically makes the solution to many diseases a software problem. Pharmaceutical companies like CureVac are working to create a Covid-19 vaccine using “messenger RNA,” which can be manually inserted into cells to initiate an immune response.
CureVac’s approach to a COVID-19 vaccine is built on mRNA technology, similar to what is being used by Moderna, Pfizer and BioNTech. It takes a small piece of messenger RNA that codes for a specific molecule on the virus’s surface. When injected into the body, it causes cells to churn out that molecule, which will train the immune system to identify the virus and neutralize it before it can replicate. No mRNA products have been approved for use yet.
The Tesla printers are being designed to be used in remote locations. They would manufacture the vaccine candidate and other potential mRNA-based therapies using whatever recipe is programmed into them.
Otherwise, CureVac is building a more typical manufacturing facility that can increase its production of the vaccine tenfold to billions of doses.

Tesla Inc is building “RNA microfactories” for coronavirus vaccine developer CureVac in Germany, the electric carmaker’s chief executive officer, Elon Musk, tweeted on Wednesday. CureVac, an unlisted German company, has said it is developing transportable, automated mRNA production units that it calls printers. They will be designed to be shipped to remote locations, where they can churn out its vaccine candidate and other mRNA-based therapies depending on the recipe fed into the machine. But for the immediate pandemic use — should its vaccine candidate win market approval — it has production sites with regulatory approval in Germany with a capacity to produce hundreds of millions of doses. It is also building a new stationary site that could increase its output tenfold to billions of doses. Musk did not elaborate on his plans. Tesla and CureVac were not immediately available to comment.
BioNTech and Pfizer announced positive results online — on MedRxiv in advance of peer review — on 20 July. This was a Phase 1/2 trial with 60 adults in Germany, and showed strong immunological responses with no ‘serious’ adverse effects (see below). This followed the first results of their US Phase 1/2 trial which were published a MedRxiv preprint published on 1 July. The news was promising there too: the vaccine triggered strong immunological responses among the 45 volunteers who received it, with more antibodies seen even than is typical in recovering COVID patients.
The Imperial College vaccine began first human trials on 24 June in the UK — it also includes an enzyme that triggers the mRNA to make more of itself, meaning smaller initial doses are needed.
Any drawbacks?
No mRNA vaccines have ever been used before, so failure is a big risk. Moderna’s Phase 1 mRNA vaccine lead to some ‘adverse events’, including fatigue, chills, headache and myalgia (these were mild to moderate, and adverse effects are not uncommon with vaccines). BioNTech’s vaccine also saw some adverse reactions “generally mild to moderate, with occasional severe events (Grade 3) of flu-like symptoms and injection site reactions”.
DNA vaccine
DNA vaccines have demonstrated strong induction of immune responses to viral pathogens in animal models, specifically in mice; however, clinical data on DNA vaccines in human subjects are limited. DNA vaccines encoding the S, N, M, and E proteins of SARS-CoV have been evaluated in mice. Vaccination with S-, M-, and N-encoding DNA vaccines induced both humoral and cellular immune responses, with some variation in the relative levels of induction.
How does it work?
No, DNA vaccines will not lead to genetically engineered humans. However, the technique does involve injecting a fragment of circular DNA, called a plasmid, into human cells. This introduced DNA codes for SARS-CoV-2 viral proteins that are then expressed by the cell and help prime the immune system to fight off an attack by COVID-19. Like mRNA, this is a new technology — no DNA vaccines have ever been fully developed and utilized in humans to prevent disease.
With the DNA vaccines, developers are testing different entry mechanisms: needle injection plus electroporation (Takis, Karolinska Institute and Inovio Pharmaceuticals) and needle-free systems (Osaka University and Immunomic Therapeutic) . The vaccines administered by the two different needle-free platforms (ActranzaTM lab and PharmaJet Tropis Needle-Free Injector System) and the Inovio Pharmaceutical candidate will be injected intradermally , and the Takis and Karolinska Institute DNA vaccine candidates will be administered by the intramuscular route .
Most DNA vaccine developers have not revealed which genes they are administering in the vaccine, but Zydus Cadila has stated they are using membrane protein. Of the SARS-CoV-2 DNA vaccines, the Immunomic Therapeutics candidate is the most unique; gene sequences will be chosen on the basis of their predicted ability to stimulate a strong immune response (selected for in collaboration with EpiVax) and will be ligated to the lysosomal-associated membrane protein gene.
Who is doing it?
The leading developer is Inovio, which worked with a DNA candidate vaccine against MERS. Several other teams are also working on DNA vaccine candidates for the novel coronavirus, including one at the Harvard Medical School.
What’s the latest?
On 30 June Inovio announced that 34 out of 36 of Phase 1 trial participants “demonstrated overall immune responses” after two doses of its INO-4800 trial vaccine. However, experts expressed caution, and some were critical of the company’s failure to disclose how many (if any) patients produced antibodies that neutralize the coronavirus, which is the important thing. Inovio discusses the use of electricity to drive a DNA-based vaccine into a cell.
Separately, the Harvard-led team announced in a paper published in Science on May 20 that various DNA candidate vaccines expressing different forms of the SARS-CoV-2 spike protein had succeeded in immunizing rhesus macaque monkeys. This adds further to hopes that at least some of these DNA vaccines will also work in humans. Some have called this a ‘moon shot’, but hey, that’s worked before…
Any drawbacks?
As with mRNA, there have never yet been DNA vaccines — and the chance that this will work first time is anyone’s guess. Inovio has been around for four decades but has yet to develop a single approved product.
Antigen Presenting Cells
- The last new vaccine platform uses artificial antigen presenting cells (APCs), pre-loaded with a COVID-19 antigen.
- The APCs then present these antigens to T and B cells that cannot detect the free or soluble antigen on their own.
- In theory, this vaccine should work more quickly since the antigen doesn’t need to be produced, then taken up by the antigen presenting cells that then have to present it to natural APCs.
- The injected artificial APCs can bypass these preliminary steps, shortening the time from vaccination to protection that is normally about a 2-week process.
- Vaccines using modified, immortalized APCs that effectively mimic human APCs are in clinical trials.Antigen-presenting cells are an essential component in the immune system’s response to a vaccine.
- Loading antigen-presenting cells with peptides that would otherwise be produced by vaccination bypasses the first steps after vaccination.
- Traditionally, dendritic cells are harvested from the individual, then expanded and manipulated to present the desired antigen, and infused back into the same individual.
- This is cost-prohibitive and too time-consuming for a vaccine deployed on a large scale.
- This has led to the development of artificial antigen-presenting cells, where immortalized cells are transduced with lentiviruses to effectively mimic antigen-presenting cells, as is the case for COVID-19/aAPC.
- Extra cold-chain requirements for a cell-based vaccine and infusion procedures hamper the deployment of these vaccines on a large scale, even more so since multiple doses are required for an efficient response.
- COVID-19 vaccines based on all next-generation platforms are currently in clinical trials; some of these have already moved from phase 1 into phase 2 or 2/3 (Fig. 1).
Vaccine requirements and challenges
- While the development of vaccines against COVID-19 is ongoing, it is important to define what we expect from this vaccine, or vaccines for future emerging viruses.
- Needless to say, the vaccine should be safe and effective, and should not induce enhanced disease upon subsequent infection, whether through vaccine-associated enhanced respiratory disease or antibody-dependent enhancement, as has been observed with certain SARS-CoV vaccines in animal models in the past.
- In order to prevent severe disease after infection, vaccination should result in either (1) complete abrogation or significant reduction of transmission within the population by the induction of herd immunity or (2) prevention of severe disease in all vaccinated individuals.
- Both approaches would require the production of large quantities of vaccine, distributed worldwide.
- A single dose vaccine that would not require a cold chain would contribute to the timeframe in which large-scale, global vaccination can be achieved.
- Ideally, vaccination would induce long-lived immunity, but annual vaccination would be feasible based on experiences with the annual influenza vaccine.
- Vaccination campaigns that either induce herd immunity or protect vaccinated individuals from severe disease face different challenges.
- Herd immunity for SARS-CoV-2 would require vaccination of ~67% of the population, which is an average and would not prevent clusters of susceptible individuals.
- However, in recent years vaccine hesitance, identified as a major threat to global health by the WHO, increased in many countries, and a recent study showed that 26% of the French population would not take a SARS-CoV-2 vaccine.
- Vaccination that does not abrogate transmission but does result in protection from severe disease seems more straightforward.
- However, two important risk groups for developing severe COVID-19, elderly (>65 years old) and obesity (body mass index > 40) have previously been linked to reduced vaccine efficacy using classical vaccination approaches.
- Whether new vaccine platforms have an increased immunogenicity in these risk groups compared to classical vaccination approaches remains to be determined.
- Besides the question of efficacy for any of the COVID-19 vaccines under development, a major hurdle will be large-scale manufacturing.
- Since the next-generation platforms, with the exception of two viral vector-based vaccines (Dengvaxia and Ervebo) are not licensed for use in humans, the feasibility to rapidly manufacture these on a large-scale is currently unclear.
- Although vaccine manufacturing capacity exists for the classical platforms, using the existing infrastructure would potentially go at the expense of regular vaccine production.
- Maintaining vaccination status for all vaccine-preventable diseases, while at the same time producing hundreds of millions of doses of COVID-19 vaccines, will be essential for global health.
- Besides the vaccine antigen itself, the required adjuvants or delivery molecules and, in the case of DNA vaccines, special delivery devices, will need to be manufactured on a mass scale as well.
- To be of use, hundreds of millions of doses need to be manufactured and distributed.
- Moreover, with the potential exception of DNA vaccines, all vaccines require a cold chain for distribution.
- Therefore, many international initiatives and investments are currently made to increase the capacity to produce and distribute vaccines.
- These collaborative programmes will be crucial for the large-scale deployment of vaccines to contain the COVID-19 pandemic; global distribution to prevent disparities in vaccination programmes between high-income countries and the rest of the world is essential in this effort.
Future directions
- The development of many of the next-generation platforms described here has so far been driven mainly by their potential use in cancer therapies.
- The COVID-19 pandemic has fast-tracked their development as vaccine platforms for emerging viruses.
- If current predictions become reality, the first vaccines against COVID-19 will be licensed within a year.
- These licensed vaccines are likely to include some of the next-generation platforms described here.
- This in itself will be a major public health achievement, yet will simultaneously result in a permanent change to the vaccine platform landscape and an increased vaccine manufacturing capacity for these novel platforms.
- Plans should be developed to ensure that the large-scale manufacturing infrastructure being built now to respond to the COVID-19 pandemic is maintained for potential future vaccine needs, as has been done for influenza vaccines.
- Once next-generation platforms are licensed, their use for other pathogens or disease indications are likely to become more easily attainable.
- Since these platforms only require sequence information to initiate vaccine development, this will increase the flexibility to adapt vaccines to antigenic changes in circulating strains, and to newly emerging viruses in general.
- The wider array of possibilities for pre-emptive and reactive vaccine design, as well as faster development and manufacturing options, will permanently change our ability to rapidly respond to emerging viruses.
- As such, the investments made now in vaccine platform development and manufacturing will pay off when we are able to respond even faster when a new virus emerges in the future.
Which vaccine will prevail?
- Which vaccine candidate will win out? It is suspected it will be whichever one gets to market first which is most likely going to be one made using one of the newer technologies.
- Sadly, we can already hear the howls of protest from antivaxxers: “Genetic engineering,” “Messing with the human genome,” “Untested, never before used, rushed to the market” and “Aborted children’s cell line used in manufacture of some COVID vaccines.”
- Some truth exists in these claims but we suspect Dr. Fauci will be one of the first in line to receive it and we will be right behind him.
COVID-19 herd immunity
- Our only way out of this pandemic crisis will be to achieve herd immunity which we fear will be difficult in the United States and other countries given the number of Americans already refusing to get this “experimental” vaccine.
- A recent Associated Press poll showed only half of Americans plan to get the vaccine.
