
HUMANS COVID19 HOPE
There was never a night or a problem that could defeat sunrise or hope. Hope shall never be endangered. Where are humans pinning hope for COVID 19 and how is hope securing human future, lets look deeper.
1. Eclipse
2. Vaccine
3. Immune System.
ECLIPSES
- NASA and various organizations had been effects of solar eclipses on the earths IONOSPHERE and relating it to know the influence of solar eclipse on nature indirectly, by analyzing certain genotypic and phenotypic variations in prokaryotes, eukaryotes , akaryotes and their impact on pathogenesis.
- Since yeast have similar gene expression as that of humans, investigations were pursued on Candida albicans. Hence the study of the effect of solar eclipse on cultures of Staphylococcus aureus, Klebsiella species, Escherichia coli,and C. albicans was performed in the laboratory. The effect of the total or partial eclipse on the microorganism isolated from clinical isolates was investigated during the time period of eclipses from 11.15 am to 3.15 pm.
- Cultures of S. aureus, Klebsiella species, and E. coli colonies on nutrient agar slants and broth and C. albicans on Sabouraud’s dextrose agar plates and broth. Slants were exposed to sunlight during eclipse and exposure to normal sunlight . There was significant change observed during exposure to normal sunlight and eclipse phase.
- Bacterial colonies showed difference in morphology on smear examination and sensitivity pattern during this study. One fungal species and three bacterial isolates were studied and changes were recorded. Fungal species showed a definite change in their morphology on exposure to sunlight during eclipse observed by stained smear examination from broth, plate, and slant.
- Study concluded that blocking of the sun rays during eclipse in some phases of the eclipse harms prokaryotes, eukaryotes and akaryotes and while in certain phases promotes the progeny of predators in the race of better acclimatization and survival in the natural and changing environmental conditions.
- The phase of eclipse observed on 26th Dec 2019 was a pomoting phase. The Ionospehere literally shut down during this phase. Coronavirus pathogenesis increased as the phenotype was heavily influenced by the environment broken by the eclipse.
- The 100s of trillions of neutrinos that passed the earth during this phase of the eclipse help the virus to mutate across species like bats, pigs , snakes and pangolins.

7. The heightened levels of pathogenesis severely impacted the human phenotype as seen in these figures.
8. It is expected that the eclipse on June 21 shall reverse the pathogenesis as the flow of neutrinos this time is of opposite polarity and will dampen the pathogenesis and make the virus inactive.
To understand that, lets look into the role of gentypes and phenotypes of COVID 19 and their roles in pathogenesis .
Genotype and Phenotype of COVID-19: Their roles in pathogenesis

COVID-19 is a novel coronavirus with an outbreak of unusual viral pneumonia in Wuhan, China, and then pandemic.
1. Coronavirus, or broadly any viruses are neither prokaryote nor eukaryote. In the classification system, cells are being classified based on nuclear and cellular structure. The virus can not be considered as a cell, rather it is just a nucleoprotein particle. viruses lack a nucleus but have instead, a central core of RNA or DNA as genetic material and protective protein envelope. Viruses depend on other organisms for their existence and are able to grow and reproduce only in the cells of other organisms. They are non-cellular and falls under the category Akaryotes.
2. Eukaryotic cells contain membrane bound organelles and includes a nucleus. Eukaryotes can be single celled or multicellular such as plants, fungi and monera etc. Bacteria are an example of prokaryotes. Prokaryotic cells do not contain a nucleus or any other membrane bound organelle. Viruses are neither prokaryotes nor eukaryotes because they lack the characteristics of living things except the ability to replicate which they accomplish only in living cells. Bacteriophages are viruses so they are neither prokaryotes nor eukaryotes.
3. Scientists estimate that there are roughly 1⁰³¹ viruses at any given moment . That’s a one with 31 zeroes after it! If you were somehow able to wrangle up all 1⁰³¹ of these viruses and line them end-to-end, your virus column would extend nearly 200 light years into space. To put it another way, there are over ten million times more viruses on Earth than there are stars in the entire universe.
4. Does that mean there are 1⁰³¹ viruses are just waiting to infect us? Actually, most of these viruses are found in oceans, where they attack bacteria and other microbes . It may seem odd that bacteria can get a virus, but scientists think that every kind of living organism is probably host to at least one virus!
5. Based on its phylogenetic relationships and genomic structures the COVID-19 belongs to genera Betacoronavirus. Human Betacoronaviruses (SARS-CoV-2, SARS-CoV, and MERS-CoV) have many similarities, but also have differences in their genomic and phenotypic structure that can influence their pathogenesis. COVID-19 is containing single-stranded (positive-sense) RNA associated with a nucleoprotein within a capsid comprised of matrix protein. A typical CoV contains at least six ORFs in its genome. All the structural and accessory proteins are translated from the sgRNAs of CoVs. Four main structural proteins are encoded by ORFs 10, 11 on the one-third of the genome near the 3′-terminus. The genetic and phenotypic structure of COVID-19 in pathogenesis is important.
Introduction
Coronaviruses are involved in human and vertebrate’s diseases. Coronaviruses are members of the subfamily Coronavirinae in the family Coronaviridae and the order Nidovirales. The recent emergence of a novel coronavirus with an outbreak of unusual viral pneumonia in Wuhan, China and then pandemic outbreak is 2019-nCoV or COVID-19. Based on its phylogenetic relationships and genomic structures the COVID-19 belongs to genera Betacoronavirus which has a close similarity of the sequences of COVID19 to that of severe acute respiratory syndrome-related coronaviruses (SARSr-CoV) and the virus uses ACE2 as the entry receptor-like SARS-CoV. These similarities of the SARS-CoV-2 to the one that caused the SARS outbreak (SARS-CoVs) the Coronavirus Study Group of the International Committee on Taxonomy of Viruses termed the virus as SARS-CoV-2. The understanding of the genetic and phenotypic structure of COVID-19 in pathogenesis is important for the production of drugs and vaccines. Hence here, we provide the newest genetic and phenotype features of COVID-19 to investigate the role of these two factors in the pathogenesis and comparing it with its families.
Coronavirus genome structure and life cycle
COVID-19 is a spherical or pleomorphic enveloped particles containing single-stranded (positive-sense) RNA associated with a nucleoprotein within a capsid comprised of matrix protein. The envelope bears club-shaped glycoprotein projections. Some coronaviruses also contain a hem agglutinin-esterase protein (HE) (Fig. 1).

Coronaviruses possess the largest genomes (26.4–31.7 kb) among all known RNA viruses, with G + C contents varying from 32% to 43%. Variable numbers of small ORFs are present between the various conserved genes (ORF1ab, spike, envelope, membrane and nucleocapsid) and, downstream to the nucleocapsid gene in different coronavirus lineages. The viral genome contains distinctive features, including a unique N-terminal fragment within the spike protein. Genes for the major structural proteins in all coronaviruses occur in the 5′–3′ order as S, E, M, and N5(Fig. 2).
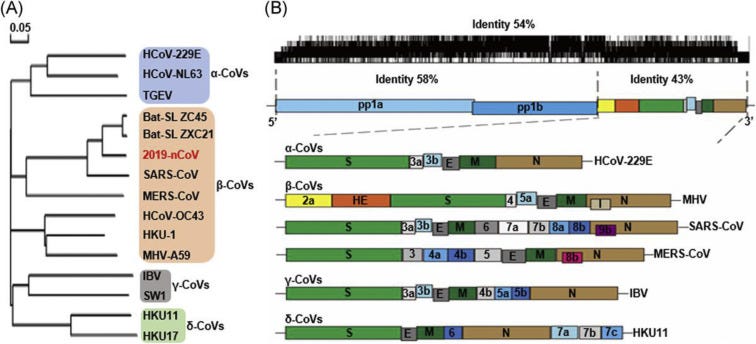
A, the phylogenetic tree of representative CoVs, with the new coronavirus COVID-19 shown in red. B, The genome structure of four genera of coronaviruses: two long polypeptides 16 nonstructural proteins have proceeded from Pp1a and pp1b represent. S, E, M, and N are represented of the four structural proteins spike, envelope, membrane, and nucleocapsid. COVID-19; CoVs, coronavirus; HE, hemagglutinin-esterase. Viral names: HKU, coronaviruses identified by Hong Kong University; HCoV, human coronavirus; IBV, infectious bronchitis virus; MHV, murine hepatitis virus; TGEV, transmissible gastroenteritis virus.
A typical CoV contains at least six ORFs in its genome. Except for Gammacoronavirus that lakes nsp1, the first ORFs (ORF1a/b), about two-thirds of the whole genome length, encode 16 nsps (nsp1–16). ORF1a and ORF1b contain a frameshift in between which produces two polypeptides: pp1a and pp1ab. These polypeptides are processed by virally encoded chymotrypsin-like protease (3CLpro) or main protease (Mpro) and one or two papain-like protease into 16 nsps. All the structural and accessory proteins are translated from the sgRNAs of CoVs. Four main structural proteins contain spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins are encoded by ORFs 10, 11 on the one-third of the genome near the 3′-terminus. Besides these four main structural proteins, different CoVs encode special structural and accessory proteins, such as HE protein, 3a/b protein, and 4a/b protein (Fig. 2B, lower panel). These mature proteins are responsible for several important functions in genome maintenance and virus replication.
There are three or four viral proteins in the coronavirus membrane. The most abundant structural protein is the membrane (M) glycoprotein; it spans the membrane bilayer three times, leaving a short NH2-terminal domain outside the virus and a long COOH terminus (cytoplasmic domain) inside the virion. The spike protein (S) as a type I membrane glycoprotein constitutes the peplomers. In fact, the main inducer of neutralizing antibodies is S protein. Between the envelope proteins with exist a molecular interaction that probably determines the formation and composition of the coronaviral membrane. M plays a predominant role in the intracellular formation of virus particles without requiring S. In the presence of tunicamycin coronavirus grows and produces spikeless, noninfectious virions that contain M but devoid of S.
Comparison of SARS-CoV2 (COVID-19), SARS-CoV, and MERS-CoV
The 5′UTR and 3′UTR are involved in inter- and intramolecular interactions and are functionally important for RNA–RNA interactions and for binding of viral and cellular proteins.8 At 5 end, Pb1ab is the first ORF of the whole genome length encoding non-structural proteins with size of 29844bp (7096aa), 29751bp (7073aa) and 30119bp (7078) in COVID-19, SARS-CoV; and MERS-CoV, respectively. Even with comparison of the spike protein at 3′ end, among the coronaviruses specifically these three betacoronaviruses, the difference was visualized, 1273aa, 21493aa, and 1270aa in COVID-19, SARS-CoV, and MERS-CoV, respectively. Genetically, COVID-19 was less similar to SARS-CoV (about 79%) and MERS-CoV (about 50%). The arrangement of nucleocapsid protein (N), envelope protein(E), and membrane protein (M) among betacoronaviruses are different as depicted in Fig. 3.9
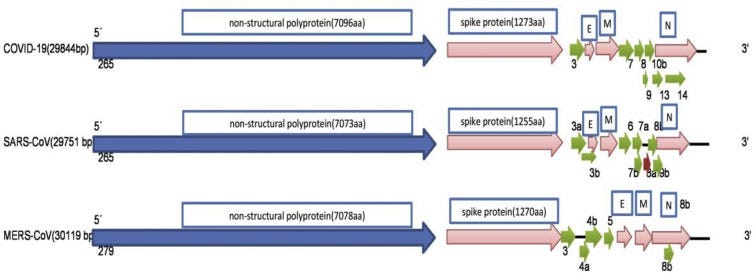
The numbers of base pairs among betacoronaviruses are shown. This figure is modified from the sequence comparison and genomic organization of 2019-nCoV, 2020.9 The differences in the arrangement of the envelope (E), membrane (M), and nucleoprotein (N) among COVID-19, SARS-CoV, and MERS-CoV are shown at 3′ end.
The role of replication process in pathogenicity
SARS-CoV-2 (COVID-19) binds to ACE2 (the angiotensin-converting enzyme 2) by its Spike and allows COVID-19 to enter and infect cells. In order for the virus to complete entry into the cell following this initial process, the spike protein has to be primed by an enzyme called a protease. Similar to SARS-CoV, SARS-CoV-2 (COVID-19) uses a protease called TMPRSS2 to complete this process. In order to attach virus receptor (spike protein) to its cellular ligand (ACE2), activation by TMPRSS2 as a protease is needed (Fig. 4).
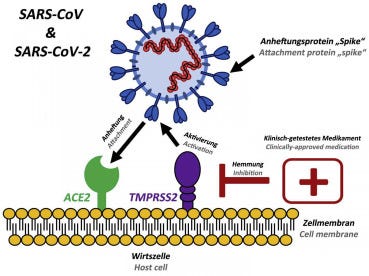
Existing, clinically approved drugs directed against TMPRSS2 inhibit SARS-CoV-2 infection of lung cells.
After the virus enters the host cell and uncoats, the genome is transcribed and then translated. Coronavirus genome replication and transcription takes place at cytoplasmic membranes and involve coordinated processes of both continuous and discontinuous RNA synthesis that are mediated by the viral replicate, a huge protein complex encoded by the 20-kb replicase gene.12 The replicase complex is believed to be comprised of up to 16 viral subunits and a number of cellular proteins. Besides RNA-dependent RNA polymerase, RNA helicase, and protease activities, which are common to RNA viruses, the coronavirus replicase was recently predicted to employ a variety of RNA processing enzymes that are not (or extremely rarely) found in other RNA viruses and include putative sequence-specific endoribonuclease, 3′-to-5′ exoribonuclease, 2′-O-ribose methyltransferase, ADP ribose 1′-phosphatase and, in a subset of group 2 coronaviruses, cyclic phosphodiesterase activities. The proteins are assembled at the cell membrane and genomic RNA is incorporated as the mature particle forms by budding from the internal cell membranes.
Factors affecting virus pathogenesis
Co-morbidities are cardiovascular and cerebrovascular disease as well as diabetes. Several abnormalities also have been observed including cellular immune deficiency, coagulation activation, myocardia injury, hepatic and kidney injury, and secondary bacterial infection. In the majority of cases of severe disease and death, lymphopenia and sustained inflammation have been recorded. Notably, these observations in COVID-19 patients are similar to those who suffered from severe acute respiratory syndrome (SARS) during the 2003 epidemic. There may be a biological mechanism behind this epidemiological anomaly.
Several kinds of vaccines and antiviral drugs that are based on S protein have been previously evaluated. Du et al. showed vaccines can be based on the S protein include full-length S protein, viral vector, DNA, recombinant S protein and recombinant RBD protein. Considering that, in the in vitro study, antiviral therapies are design based on S protein include RBD–ACE2 blockers, S cleavage inhibitors, fusion core blockers, neutralizing antibodies, protease inhibitors, S protein inhibitors, and small interfering RNAs. There are some recombinant compounds such as IFN with ribaverin which has only limited effects against COVID-19 infection. The receptor-binding domain of SARS-CoV-2 has a higher affinity for ACE2, while it is a lower affinity for SARS-CoV.
Angiotensin-converting enzyme (ACE) and its homologue ACE2, belongs to the ACE family of dipeptidylcarboxy dipeptidase. However, their physiological functions are varied. On the other hand, ACE2 serves as the binding site for COVID-19. Based on this information, Gurwitz suggested using available angiotensin receptor 1 (AT1R) blockers, such as losartan, as therapeutics for reducing the severity of COVID-19 infections.
At present therapy is based on identifying and developing monoclonal antibodies that are specific and effective against COVID-19 combines with remdesivir as a novel nucleotide analog prodrug that was used for the treatment of the Ebola virus disease.
To understand the rate of virus spread among people, it is crucial to figure out whether COVID-19 is mutating to improve its binding to human receptors for infection considering its high mutation rate. Any adaptation in the COVID-19 sequence that might make it more efficient at transmitting among people might also boost its virulence. The COVID-19 is expected to become less virulent through human to human transmissions due to genetic bottlenecks for RNA viruses often occur during respiratory droplet transmissions.
Inference
At present, all treatements are trying to identify the basis of its replication, structure, and pathogenicity for discovering a way to the special treatment or the prevention. Due to the high similarity of the virus to its families, efforts have been made to provide medicines and vaccines for COVID-19. Differences in the length of the spike as it is longer in COVID-19 are likely to play an important role in the pathogenesis and treatment of this virus. However, identifying the specific molecular details of the virus is helpful in achieving treatment goals.
Vaccine Development
— The University of Oxford in the UK has reported positive findings of its Covid-19 vaccine candidate in a small preclinical study involving six monkeys.
This preliminary data of the vaccine, currently in human trials, has been published on the preprint website bioRxiv, noted Reuters.
According to the publication, some monkeys administered with a single shot of the vaccine generated antibodies against the virus within 14 days while all monkeys developed antibodies within 28 days, prior to being exposed to high virus doses.
Following exposure to the coronavirus, the vaccine candidate was prevented damage to the lungs and blocked the virus from replication. However, the virus was found to be actively replicating in the nose.
London School of Hygiene & Tropical Medicine pharmacoepidemiology professor Stephen Evans said that the animal data was ‘good news’. Evans was quoted as saying: “It is one of the hurdles to be passed by the Oxford vaccine and it has cleared it well.”
The professor also noted that no evidence of immune-enhanced disease, where a vaccine makes the disease worse, was reassuring.
Evans added: “This was a definite theoretical concern for a vaccine against SARS-CoV-2 and finding no evidence for it in this study is very encouraging.”
Last month, the University of Oxford signed an agreement with AstraZeneca to develop and distribute its Covid-19 vaccine candidate, a recombinant adenovirus vaccine in development by Jenner Institute and Oxford Vaccine Group at the university.
Results from the human trial are expected next month, with plans to start late-stage trials by the middle of this year. As part of the deal, AstraZeneca will carry out the development and global manufacturing and distribution of the vaccine.
Director of the National Institute of Allergy and Infectious Disease Dr. Anthony Fauci said he expects the US will have “hundreds of millions of doses” of vaccine ready to deploy by early 2021 in a June 2 interview with Journal of the American Medical Association Editor in Chief Dr. Howard Bauchner.
Fauci said he is personally working with four or five different vaccine projects that are either in human trials or about to be. One of those projects, spearheaded by Massachusetts-based biotech company Moderna, has announced it will enter broad Phase 3 clinical trials this July, which could include as many as 30,000 human participants, according to Fauci. Other vaccine candidates, including those from Oxford University and AstraZeneca, Pfizer and Chinese vaccine developer Sinovacaren’t far behind.
The presumed frontrunners: Moderna and Oxford University
Moderna has been making headlines for its coronavirus vaccine development — both positive and negative. Early reports that Moderna’s first trials showed promise for immunity caused Moderna’s stock to soar. Soon after, however, scientists cast doubt on the company’s data, causing the same stocks to falter.
Moderna is a beneficiary of the US Food and Drug Administration’s program to fast-track vaccines. The fast-track process expedites approval by allowing select labs to submit their review process by phases, rather than submitting all sections of the application at once, which is the usual way. The company ran Phase 1 clinical trials and reported preliminary data that it says supports the move to a larger Phase 2 trial, which is currently ongoing. Phase 3 is reportedly slated for July. You can learn more about Moderna’s vaccine candidate, mRNA-1273.
Another vaccine is under development at Oxford University in the UK. Scientists there say that vaccine could be ready by the fall of 2020. Oxford is working with pharmaceutical giant AstraZeneca. Their vaccine candidate is slated to begin simultaneous Phase 2 and Phase 3 trials this month.
Scientists say in a paper that results from Oxford’s trials on mice and rhesus monkeys are mixed, however, speculating that humans who eventually take the vaccine might still be able to spread the virus. You can read more about this effort, called ChAdOx1 nCoV-19, at AstraZeneca’s website.
Will there be just one vaccine for everyone?
We won’t know for a long time, but Fauci has suggested that it might take several different vaccines made and distributed by different labs in order to effectively eradicate COVID-19 from the planet. Fauci co-authored a paper about vaccines published May 11 in the journal Science.
What else is happening with coronavirus vaccine development
There are currently over 100 vaccines reportedly under development in countries around the world, including the US, UK, Germany, Japan and China, with 13 reportedly already in clinical trials as of last month. That means there are more scientists working harder and faster on finding a vaccine than ever before in the history of pandemics. But even if one or more of the vaccines now in the works turns out to be effective, the FDA approval process typically takes a year or longer.
This April, the White House began organizing “Operation Warp Speed,” according to Bloomberg, a sort of coronavirus vaccine task force that has identified 14 vaccine projects that it will focus on fast-tracking. The “Warp Speed” project itself, which the White House acknowledged during an April press briefing, has a stated goal of readying 300 million doses of vaccine to be available by January 2021, which coincides with Fauci’s estimation.
How good are the odds for finding a vaccine?
Statistically, only about 6% of vaccine candidates ever make it through to market, according to a Reuters special report, and not just because they don’t work. There’s a whole litany of problems that could cancel even a promising candidate.
Take, for example, what happened when scientists tried to develop a vaccine for SARS — it backfired and actually made people more susceptible to the disease. The same thing happened with a vaccine for Dengue fever. To make matters worse, coronaviruses are a large class of viruses and so far there are no vaccines for any of them.
However, this particular coronavirus, SARS-CoV-2, has some unique traits that may help researchers working on a vaccine. For example, some viruses, like the flu, mutate quickly and often, which is why there’s a new flu vaccine every year. Early evidence suggests that the coronavirus doesn’t appear to do that. Although some researchers have hypothesized that a more contagious strain has developed, others aren’t so sure. Either way, it’s thought that the virus has not yet mutated significantly enough to disrupt vaccine development, nor is it expected to, though it’s too soon to say for certain, and there are still many unknowns about the virus’ behavior.
What steps do vaccines have to go through to get approved?
Rules and regulations vary by country, but, generally speaking, most industrialized nations have similar protocols for approving a vaccine. The following path is how vaccines are approved in the US under the FDA:
- Before clinical trials can begin: Once a laboratory has researched and developed a potential vaccine, which includes testing it in animal models and working out manufacturing and quality control processes, it can apply to the FDA to start clinical trials.
Phase 1 clinical trials: The vaccine is tested for safety and effectiveness in a small number (dozens) of closely monitored subjects.
Phase 2 clinical trials: Various dosages of the vaccine are tested on hundreds of human subjects.
Phase 3 clinical trials: Thousands of subjects are enrolled to measure the overall effectiveness of the vaccine.
If a vaccine passes all three phases: The lab must then apply to the FDA for a license to produce and distribute the vaccine. That application is reviewed by both FDA and non-FDA scientists.
If approved: The lab begins producing the vaccine while the FDA closely monitors production.
Phase 4: Although at this point the vaccine may be released to the market, many vaccines continue with what’s called Phase 4 studies, during which the FDA continues to review the safety and efficacy of the vaccine.
What happens if we never find a coronavirus vaccine?
The longer we go without a vaccine, the more likely focus will shift toward treatments, such as the experimental antiviral drug remdesivir, which has reportedly shown promising results. With effective therapeutic treatments, many viruses that used to be fatal are no longer death sentences. Patients with HIV, for example, can now expect to enjoy the same life expectancy as non-HIV-positive individuals, thanks to tremendous advances in treatment.
Without a coronavirus vaccine, the road back to “normal life” may be harder and longer, but not necessarily impossible. Coronavirus testing, including antibody testing, and contact-tracing efforts would need to intensify, experts say.
Lockdown measures are already lifting throughout the world, although with a potential second wave of coronavirus infections, cities could bring back certain quarantine measures, including requiring face masks and social distancing. Eventually, the global population may reach the 60% to 70% rate required for herd immunity to protect those who aren’t immune.
Searching for effective treatments
Drug development is sometimes described as a pipeline, with compounds moving from early laboratory development to laboratory and animal testing to clinical trials in people.
It can take a decade or more for a new compound to go from initial discovery to the marketplace. Many compounds never even make it that far.
That’s why many medications being eyed as potential treatments for COVID-19 are drugs that already exist.
In a recent review in the British Journal of Pharmacology, scientists from the United Kingdom called for wider screening of existing drugs to see if they might work against the coronavirus.
They identified three stages of infection at which the coronavirus could be targeted: keeping the virus from entering our cells, preventing it from replicating inside the cells, and minimizing the damage that the virus does to the organs.
Many of the drugs being developed or tested for COVID-19 are antivirals. These would target the virus in people who already have an infection.
Lee says antivirals work better if you administer them sooner, “before the virus has a chance to multiply significantly.” And also before the virus has caused significant damage to the body, such as to the lungs or other tissues.
Dr. Robert Amler, dean of the School of Health Sciences and Practice at New York Medical College and a former chief medical officer at the Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry (ATSDR), says both antivirals and vaccines will be valuable tools in combating COVID-19.
However, he told Healthline that “antivirals are likely to be developed and approved before a vaccine, which typically takes longer.”
- Scientists around the world are working on potential treatments and vaccines for the new coronavirus disease known as COVID-19.
- Several companies are working on antiviral drugs, some of which are already in use against other illnesses, to treat people who already have COVID-19.
- Other companies are working on vaccines that could be used as a preventive measure against the disease.
With confirmed COVID-19 cases worldwide surpassing 6.4 million and continuing to grow, scientists are pushing forward with efforts to develop vaccines and treatments to slow the pandemic and lessen the disease’s damage.
Some of the earliest treatments will likely be drugs that are already approved for other conditions, or have been tested on other viruses.
“People are looking into whether existing antivirals might work or whether new drugs could be developed to try to tackle the virus,” said Dr. Bruce Y. Lee, a professor at the CUNY Graduate School of Public Health & Health Policy.
HEALTHLINE’S CORONAVIRUS COVERAGE
Stay informed with our live updates about the current COVID-19 outbreak.
Also, visit our coronavirus hub for more information on how to prepare, advice on prevention and treatment, and expert recommendations.
As of May 8, three medicationsTrusted Source had received emergency use authorization (EUA) from the Food and Drug Administration (FDA) — the anti-malaria drugs chloroquine and hydroxychloroquine, the anti-viral remdesivir, and a drug used to sedate people on a ventilator.
An EUA allows doctors to use these drugs to treat people with COVID-19 even before the medications have gone through the formal FDA approval process.
In mid-May, the small biotech company, Sorrento Therapeutics, announced it has an antibody drug that has been effective in early testing in blocking the virus that causes COVID-19. They say the drug could potentially be used to treat people with COVID-19 as well as help prevent infection.
These drugs are still being tested in clinical trials to see if they are effective against COVID-19. This step is needed to make sure the medications are safe for this particular use and what the proper dosage should be.
So it could be months before treatments are available that are known to work against COVID-19. It could be even longer for a vaccine.
But there are still other tools we can use to reduce the damage done by the novel coronavirus.
“Even though technological advances allow us to do certain things more quickly,” Lee told Healthline, “we still have to rely on social distancing, contact tracing, self-isolation, and other measures.”
Searching for effective treatments
Drug development is sometimes described as a pipeline, with compounds moving from early laboratory development to laboratory and animal testing to clinical trials in people.
It can take a decade or more for a new compound to go from initial discovery to the marketplace. Many compounds never even make it that far.
That’s why many medications being eyed as potential treatments for COVID-19 are drugs that already exist.
In a recent review in the British Journal of Pharmacology, scientists from the United Kingdom called for wider screening of existing drugs to see if they might work against the coronavirus.
They identified three stages of infection at which the coronavirus could be targeted: keeping the virus from entering our cells, preventing it from replicating inside the cells, and minimizing the damage that the virus does to the organs.
Many of the drugs being developed or tested for COVID-19 are antivirals. These would target the virus in people who already have an infection.
Lee says antivirals work better if you administer them sooner, “before the virus has a chance to multiply significantly.” And also before the virus has caused significant damage to the body, such as to the lungs or other tissues.
Dr. Robert Amler, dean of the School of Health Sciences and Practice at New York Medical College and a former chief medical officer at the Centers for Disease Control and Prevention (CDC) Agency for Toxic Substances and Disease Registry (ATSDR), says both antivirals and vaccines will be valuable tools in combating COVID-19.
However, he told Healthline that “antivirals are likely to be developed and approved before a vaccine, which typically takes longer.”
CORONAVIRUS UPDATES
Stay on top of the COVID-19 outbreak
We’ll email you once a day as our news team publishes new and updated information about the novel coronavirus, including case counts and treatment information.
Enter your email
SIGN UP NOW
Your privacy is important to us
Antivirals
Remdesivir
Developed a decade ago, this drug failed in clinical trials against Ebola in 2014. But it was found to be generally safe in people. Research with MERS showed that the drug blocked the virus from replicating.
The drug is being tested in many COVID-19 clinical trials around the world. This includes studies in which remdesivir is being administered alongside other drugs, such as the anti-inflammatory drug baricitinibTrusted Source.
In late April, the drug’s manufacturer Gilead Sciences announced one of its trials had been “terminated” due to low enrollment. Gilead officials said the results of that trial had been “inconclusive” when it was ended.
A few days later, the company announced that preliminary data from another trial of remdesivir overseen by the National Institute of Allergy and Infectious Diseases (NIAID) had “met its primary endpoint.”
Dr. Anthony FauciTrusted Source, the institute’s director, told reporters the trial produced a “clear cut positive effect in diminishing time to recover.” He said people taking the drug recovered from COVID-19 in 11 days compared to 15 days for people who didn’t take remdesivir. More details will be released after the trial is peer reviewed and published.
Gary Schwitzer, founder of HealthNewsReview.org, though, said that the researchers changed the primary endpoint two weeks before Fauci’s announcement. Schwitzer compared that to moving football goalposts closer to make it easier to get a touchdown.
At the same time, another studyTrusted Source published in The Lancet reported that participants in a clinical trial who took remdesivir showed no benefits compared to people who took a placebo.
Despite the conflicting results, the FDA issued an orderTrusted Source on May 1 for the emergency use of remdesivir.
In early June, federal officials announced their supply of remdesivir will run out by the end of June. Gilead is ramping up production, but it’s unclear how much of the drug will be available this summer.
Kaletra
This is a combination of two drugs — lopinavir and ritonavir — that work against HIV. Clinical trials are being done to see whether it also works against SARS-CoV-2.
One small study published May 4 in the journal Med by Cell Press found that lopinavir/ritonavir did not improve outcomes in people with mild or moderate COVID-19 compared to those receiving standard care.
Another study, published May 7 in the New England Journal of Medicine, found that the drug combination was not effective for people with severe COVID-19.
But another study found that people who were given lopinavir/ritonavir along with two other drugs — ribavirin and interferon beta-1b — took less time to clear the virus from their body. This study was publishedTrusted Source May 8 in The Lancet.
Favipiravir
This drug is approved in some countries outside the United States to treat influenza. Some reports from China suggest it may work as a treatment for COVID-19. These results, though, haven’t been published yet.
Japan, where the medication is made, is sending the drug to 43 countries for clinical trial testing in people with mild or moderate COVID-19.
Arbidol
This antiviral was tested along with the drug lopinavir/ritonavir as a treatment for COVID-19. Researchers reported in mid-April that the two drugs didn’t improve the clinical outcomes for people hospitalized with mild to moderate cases of COVID-19.
HEALTHLINE RESOURCES
Until you get through this, count on our support
In difficult times, you need to be able to turn to experts who understand and can help strengthen your mental well-being. We’re here for you.
Other treatments
Scientists are also looking at other ways to target the virus or treat the complications of COVID-19.
Among them is ibuprofen.
In early June, scientists started a clinical trial to see if the pain medication could be used on people hospitalized with COVID-19.
Their theory is ibuprofen’s anti-inflammatory qualities could help ease breathing difficulties associated with the illness.
Hydroxychloroquine and chloroquine
These drugs received emergency use authorization from the FDA at the end of March.
On June 15, the FDA revoked that authorization, citing studies that indicated hydroxychloroquine didn’t significantly help people with COVID-19 and may have caused serious health risks.
At the time of the FDA authorization in March, manufacturer Novartis donated about 30 million doses of hydroxychloroquine and one million doses of chloroquine to the nation’s Strategic National Stockpile.
Clinical results since then have been, at best, mixed.
In late May, the World Health Organization announced it was halting its clinical trials of hydroxychloroquine due to safety concerns.
Around the same time, officials in France, Belgium, and Italy stopped the use of hydroxycholoroquine for COVID-19 treatment due to safety concerns.
In early May, the authors of an observational study in the New England Journal of Medicine reported that patients who had been given hydroxychloroquine didn’t benefit. The medication didn’t harm the participants who took it, but the drug also didn’t lessen their need for ventilators or reduce their risk of death.
Another studyTrusted Source in JAMA published in May also found that hydroxychloroquine, with or without the antibiotic azithromycin, didn’t help people with COVID-19.
Given the scarcity of good data, the authors of an opinion pieceTrusted Source in the journal Annals of Internal Medicine questioned the use of these medications.
In late April, the FDA issued a warningTrusted Source against the use of both hydroxychloroquine and chloroquine outside of medical facilities. The agency stated there were “serious and potentially life threatening heart rhythm problems” connected with the drugs.
Monoclonal antibodies
These drugs trigger the immune system to attack the virus.
Vir Biotechnology has isolated antibodies from people who survived SARS. The company is working with Chinese firm WuXi Biologics to test them as a treatment for COVID-19.
AbCellera has isolated 500 unique antibodies from a person who recovered from COVID-19 and is set to start testing them.
Blood plasma transfers
Along the same lines, the FDA has announcedTrusted Source a process for medical facilities to conduct trials on an experimental treatment that uses blood plasma from people who have recovered from COVID-19.
The theory is that the plasma contains antibodies that will attack this particular coronavirus. In late March, the New York Blood Center began collecting plasma from people who have recovered from COVID-19.
In early June, researchers reported that 19 of 25 people with COVID-19 who were treated with convalescent plasma transfusions at Houston Methodist Hospital in Texas had improved. Eleven of those patients have been released from the hospital.
Stem cells
Athersys Inc. began a phase II/III clinical trial that will examine whether the company’s stem cell treatment could potentially benefit people with acute respiratory distress syndrome (ARDS). This condition occurs in some people with severe COVID-19.
Mesoblast has also developed a potential stem cell treatment for ARDS. The company is enrolling people with moderate to severe ARDS into a phase II/III clinical trial in the United States.
Immune suppressants
In some people with COVID-19, the immune system goes into overdrive, releasing large amounts of small proteins called cytokines. Scientists think this “cytokine storm” may be the reason certain people develop ARDS and need to be put on a ventilator.
Several immune suppressants are being tested in clinical trials to see whether the drugs can quell the cytokine storm and reduce the severity of ARDS.
These include baricitinib, a drug for rheumatoid arthritis; CM4620-IE, a drug for pancreatic cancer; and IL-6 inhibitors. The FDA has also approved a device that filters cytokinesTrusted Source out of the blood of patients.
Next steps for treatments
While a lot of the focus is on developing new treatments for COVID-19, improvements in how doctors care for patients using existing technology are also crucial.
“The things that we have to worry about with the novel coronavirus is that it can cause pneumonia and acute respiratory distress syndrome,” Lee said. “There are ways of treating those things that can reduce the effects, so doctors are trying to use those as well.”
No company has offered a timeline for when its drug might be used more widely to treat COVID-19. This isn’t an easy thing to estimate.
After laboratory and animal testing, drugs have to pass through several clinical trial stagesTrusted Sourcebefore they can be approved for widespread use in people.
It’s also difficult to speed things up, because scientists have to enroll enough people in each stage to have useful results. They also have to wait long enough to see whether there are harmful side effects of the drug.
However, drugs can sometimes be given to people outside a clinical trial through the FDA’s “compassionate useTrusted Source” program. For this to happen, people must have an “immediately life threatening condition or serious disease or condition.”
Doctors at the University of California, Davis were able to secure this type of approval for a woman with severe COVID-19 to receive remdesivir. They reported she was doing well.
Many will take this as a sign that the drug works. But because the drug was given outside of a clinical trial to just one person, it’s not possible to know for certain. Also, other people may not have the same response to the drug.
Improvements in testing can also reduce COVID-19 deaths by slowing the spread of the virus. As cities and states lift stay-at-home and physical distancing orders, increased testing will be needed to prevent large spikes in infections.
The FDA has granted emergency use authorizations for many diagnostic tests for coronavirusTrusted Source. Companies and universities around the world also continue to develop new ones.
On May 8, the FDA announced the authorization of the first at-home saliva-based COVID-19 diagnostic test.
The test, which was designed by Rutgers Clinical Genomics Laboratory, allows people to spit in a tube at home and mail it back to the Rutgers lab for testing.
This is the first at-home test approved involving saliva collection — all other approved at-home tests are conducted via a nasal swab.
The Rutgers test will hopefully expand access to people unable to easily make it to a clinic or drive-by testing facility. The test is currently only available by prescription.
New guidelines were posted by the FDA in early May designed to expedite the development and approval of more at-home self-collection kits to further expand access to testing.
Under the new guidance, test developers are encouraged to reach out to the FDA to ensure their kits and shipping methods are in compliance with the most up-to-date regulations.
One commercially-available test developed by scientists in Europe can show in 15 minutes whether someone is infected with coronavirus. The test uses a sample collected with a nasopharyngeal swab inserted into the nose.
A real-world analysis found that the test could detect 6 out of 10 people with coronavirus infection. It performed much better at identifying when an infection was not present.
While the test is not 100 percent effective, it doesn’t require special reagents or trained laboratory staff to run. This would make it ideal for health clinics or in low- and middle-income countries with few clinical laboratories.
But it would have to be part of a broader testing strategy.
Vaccines
A vaccine is designed to protect people before they’re exposed to a virus — in this case, SARS-CoV-2, the virus that causes COVID-19.
A vaccine basically trains the immune systemTrusted Source to recognize and attack the virus when it encounters it.
Vaccines protect both the person who’s vaccinated and the community. Viruses can’t infect people who are vaccinated, which means vaccinated people can’t pass the virus to others. This is known as herd immunity.
Many groupsTrusted Source are working on potential vaccines for SARS-CoV-2, with several backed by the nonprofit Coalition for Epidemic Preparedness Innovations (CEPI).
There are more than 100 projectsTrusted Source around the world centered on the development of a vaccine for the coronavirus. As of May 11, eight candidate vaccines were being tested in clinical trials in people.
An official at the National Institutes of Health said in mid-May that large-scale testing could begin in July with a vaccine potentially available by January.
Other experts say the more likely timeline is summer or fall of 2021.
Here’s a look at some of the projects:
- Moderna. In March, the company began testing its messenger RNA (mRNA) vaccine in a phase I clinical trial in Seattle, Washington. In mid-May, the company announced the vaccine had produced antibodies in all 45 trial participants in this initial clinical phase. The study included 45 healthy volunteers, ages 18 to 55, who are getting two shots 28 days apart. The company has developed other mRNA vaccines before. Those earlier studies showed that their platform is safe, which allowed the company to skip certain animal testing for this specific vaccine. In early May, the company received permission from the FDA to start a phase II study of its vaccine. The company expects to begin a phase III clinical trial in July. The FDA also agreed to fast-track regulatory review of this vaccine if it succeeds in a phase III clinical trial.
- Inovio. When COVID-19 appeared in December, the company had already been working on a DNA vaccine for MERS, which is caused by another coronavirus. This allowed the company to quickly develop a potential vaccine for SARS-CoV-2. Company officials announced at the end of April that it had enrolled 40 healthy volunteers in its phase I clinical trial. It is preparing to start a phase II/III clinical trial this summer.
- University of Oxford in England. A clinical trial with more than 500 participants began in late April. Oxford officials said the potential vaccine has an 80 percent chance for success and could be available as early as September. The vaccine uses a modified virus to trigger the immune system. The university has partnered with pharmaceutical company AstraZeneca. The company reported in mid-May the vaccine was effective against COVID-19 after it was given to six rhesus macque monkeys. The company expects to begin a late-stage clinical trial by the middle of this year. Officials said in mid-May that if the clinical trial is successful, they could deliver 30 million doses by September.
- University of Queensland in Australia. Researchers are developing a vaccine by growing viral proteins in cell cultures. They began preclinical testing stages in early April.
- Pharmaceutical companies. Johnson & Johnson and Sanofi are both working on a vaccine of their own. Johnson & Johnson announced on June 10 that they will begin early-stage human clinical trials in late uly. Pfizer has also teamed up with a German company to develop a vaccine. Their initial clinical trial with 200 participants was given the green light in late April. The two companies began human testing in the United States in early May.
Advances in genetic sequencing and other technological developments have sped up some of the earlier laboratory work for vaccine development.
However, Fauci told reporters in March that a vaccine won’t be available for widespread use for at least another 12 to 18 months.
This is the timeline to complete the phase III clinical studies.
Fauci wrote in the journal Science in May that multiple successful vaccines may be needed to meet the demand of vaccinating billions of people worldwide.
Speeding up vaccine development
Some scientists argue that a “human challenge trial” could speed up the vaccine clinical trials — potentially shaving months off the timeline.
In this type of trial, healthy volunteers are given a potential vaccine and then intentionally infected with the coronavirus.
Usually, researchers wait for a person given a test vaccine to contract the virus naturally. Then they look at how well the person was protected by the vaccine.
There are no plans for this kind of study in the United States, but more than 16,000 people in more than 100 countries have signed up to take part.
A human challenge trial raises many ethical questions. One is that there’s still a lot we don’t know about the coronavirus, including who will get very ill or die from COVID-19.
That means people can’t really know the risks of participating in the study, so they wouldn’t be able to give high-quality informed consent. This is an essential part of modern clinical trials.
Given the scope of the pandemic, though, some experts think this type of trial will happen eventually.
In preparation for this, the World Health Organization recently released ethical guidelines to navigate these tricky waters.
Meanwhile, some clinical trials are underway in the Netherlands and Australia to see whether existing vaccines for tuberculosis might also protect against SARS-CoV-2.
The polio vaccine is another possible option. Scientists think these vaccines might boost the immune system just enough to fight off the new coronavirus, although there’s no evidence yet to confirm this theory.
There’s no guarantee any of the vaccine candidates will work.
“There’s a lot of uncertainty with vaccine development,” Lee said. “Naturally, you have to make sure the vaccine is safe. But you also have to make sure the vaccine will elicit enough of an immune response.”
Like drugs, potential vaccines have to pass through the same clinical trial stagesTrusted Source. This is especially important when it comes to safety, even during a pandemic.
“The public’s willingness to back quarantines and other public-health measures to slow spread tends to correlate with how much people trust the government’s health advice,” Shibo Jiang, a virologist at Fudan University in China, wrote in the journal NatureTrusted Source.
“A rush into potentially risky vaccines and therapies will betray that trust and discourage work to develop better assessments,” he said.
Clinical trial stages
- Phase I. The drug is given to a small number of healthy people and people with a disease to look for side effects and figure out the best dose.
- Phase II. The drug is given to several hundred people who have the disease, looking to see whether it works and if there are any side effects that weren’t caught during the initial testing.
- Phase III. In this large-scale trial, the drug is given to several hundred or even up to 3,000 people. A similar group of people take a placebo, or inactive compound. The trial is usually randomized and can take 1 to 4 years. This stage provides the best evidence of how the drug works and the most common side effects.
- Phase IV. Drugs that are approved for use undergo continued monitoring to make sure there are no other side effects, especially serious or long-term ones.
With more than 160 potential vaccines for COVID-19 under study, optimistic experts hope that a viable vaccine may be ready by the end of 2020.
Other experts caution that the timeline may be unrealistic. Only a small number of those vaccine candidates are being tested on people, and chances are many of the other projects won’t survive beyond the laboratory stage.
Dos and Don’ts of Disinfectants
Can a DIY cleaner made with vodka kill coronavirus bacteria? Here’s what you should know.
ABOUT
Even so, vaccine experts point out that funding has been plentiful, many different approaches are under study, and collaborations between small firms developing the vaccines and large drug companies with the capacity to mass produce them all give reason for hope.
The U.S. said it would fund and conduct the phase III trials — the final step to determine how well the vaccine works and if it’s safe — of three candidates: Moderna Inc., AstraZeneca, and Johnson & Johnson. The Moderna and AstraZeneca vaccines are already being tested in people, while J&J announced Wednesday it will begin its testing in the second half of July.
Here are some that are furthest along, with details on how the vaccine works.
Moderna. Moderna’s vaccine, mRNA-1273, uses messenger RNA, an approach that does not require a virus to make the vaccine. The messenger RNA, or mRNA, carries instructions for making the spike protein, a key protein on the surface of the SARS-CoV-2 virus that allows the virus to enter cells when a person gets infected. When the vaccine with this instruction molecule is injected, it goes to the immune cells and instructs them to make copies of the spike protein, acting as if the cells have been infected with the coronavirus. Allowing other immune cells to develop ways to protect you gives immunity.
mRNA-1273 is in phase II of its clinical trial, designed to evaluate safety and effectiveness. Moderna, a biotechnology company working with the National Institute of Allergy and Infectious Diseases, intends to enroll 600 healthy volunteers equally divided into two age groups: 18 to 55, and 55 and older. The company announced on June 11 that it will start phase III of its trial in July with 30,000 volunteers. Phase III, the final clinical trial phase, evaluates effectiveness in a much larger group and compares how well the vaccine works compared to a placebo. Moderna will test a 100 microgram dose and said the company is on track to deliver 500 million doses per year. In mid-May, the company announced that all eight initial trial volunteers given two different dose amounts reached or surpassed the level of antibodies capable of neutralizing the virus.
University of Oxford and AstraZeneca. University of Oxford scientists are partnering with AstraZeneca to develop a COVID-19 vaccine made from a weakened version of a common cold virus, the adenovirus, taken from chimpanzees. The adenovirus is genetically altered so it can’t reproduce itself. The vaccine is combined with genes of the spike protein to trigger production of vaccines against it that allows the immune system to destroy the SARS-CoV-2 virus.
A phase I/II clinical trial began in April in the U.K. to assess its safety and how well it works in more than 1,000 healthy volunteers 18 to 55 years old. Now, recruiting has begun for phase II/III trials, which will enroll up to 10,260 adults and children. For both phase II and III, volunteers will receive one or two doses of either the COVID-19 vaccine or a licensed vaccine that will be used as a control for comparison. In early June, Brazil, hard hit with COVID-19 cases, joined the clinical trials, planning to test 2,000 volunteers there.
After reaching a license agreement with Oxford University and others, AstraZeneca agreed to supply more than 2 billion doses globally, anticipating delivery of 400 million doses before the end of 2020.
Pfizer and BioNTech. The companies are testing four vaccines, each using messenger RNA, with a different combination of mRNA to targeted antigens (to produce antibodies). Called BNT162, volunteers in Germany and the U.S. have received the vaccine in a phase I/II clinical trial. This trial will evaluate the safety, ability to give immunity, and the optimal dose of the four candidates in a single and continuous study. Initially they are testing the vaccine on people 18 to 55. Once a given dose level is proven safe and effective, older adults will be immunized. Pfizer is predicting the production of millions of vaccine doses in 2020, increasing to hundreds of millions in 2021. Manufacturing sites have been identified both in the U.S. and elsewhere.
Inovio. Inovio’s vaccine, INO-4800, is a DNA vaccine in phase I clinical trials, with 40 volunteers. The technology uses DNA designed to produce a specific immune response. A handheld smart device uses a brief electrical pulse to open small pores in the skin to deliver the vaccine. Once the DNA is inside a cell, it instructs it to make many copies of the artificial DNA, and this stimulates the body’s natural immune response.
Results from the U.S. phase I trial are expected in June, and a phase II/III trial is expected then to begin. Human trials are also expected to begin this summer in China and South Korea. Multiple partners and collaborators are involved, including the Bill & Melinda Gates Foundation, the National Institutes of Health, and others.
CanSino. CanSino Biologics in Tianjin, China, is working with the Beijing Institute of Biotechnology on a coronavirus vaccine using a type of genetically altered adenovirus known as Ad-5. The platform has been used successfully to develop the Ebola virus vaccine.
In late May, researchers reported on results of the phase I safety study, in which 108 people got three doses (low, middle, high) of the vaccine. Most volunteers developed immune responses, but fewer had the neutralizing antibodies experts say are crucial to fight off the virus.
The company launched phase II in mid-April, with over 500 enrolled.
Sinovac Biotech. Sinovac Biotech’s vaccine, CoronaVac, uses an inactivated version of the virus. Early results of a Phase II clinical trial released in June show that the vaccine induced antibodies to neutralize the virus after 14 days in 90% of people who received it. The vaccine requires two injections, given two weeks apart, according to the company. No serious side effects have been reported in either phase I or II trials, which included 743 healthy volunteers.
Sinovac will partner with Instituto Butantan in Brazil to launch a phase III trial. The company said it will develop the vaccine for global use.
Johnson & Johnson. The company said it expected to start testing its vaccine in people in the second half of July. The vaccine combines genes from the coronavirus with a modified adenovirus. The first trial will include more than 1,000 healthy adults aged 18 to 55 and others 65 and older, and will take place in the U.S. and Belgium.
Other efforts. The Trump administration chose five companies for Operation Warp Speed, the national program to accelerate the development, making, and distribution of COVID-19 vaccines, treatments, and diagnostics. They are: Moderna, Johnson & Johnson, Merck, Pfizer and BioNTech, and AstraZeneca/Oxford University.
